Preparation method of high-purity iridium dioxide
An iridium dioxide, high-purity technology, applied in chemical instruments and methods, ruthenium/rhodium/palladium/osmium/iridium/platinum oxide/hydroxide, inorganic chemistry, etc., can solve the problem of low purity of iridium dioxide, difficult Purification and other issues to achieve the effect of eliminating complex processes, reducing economic costs, and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
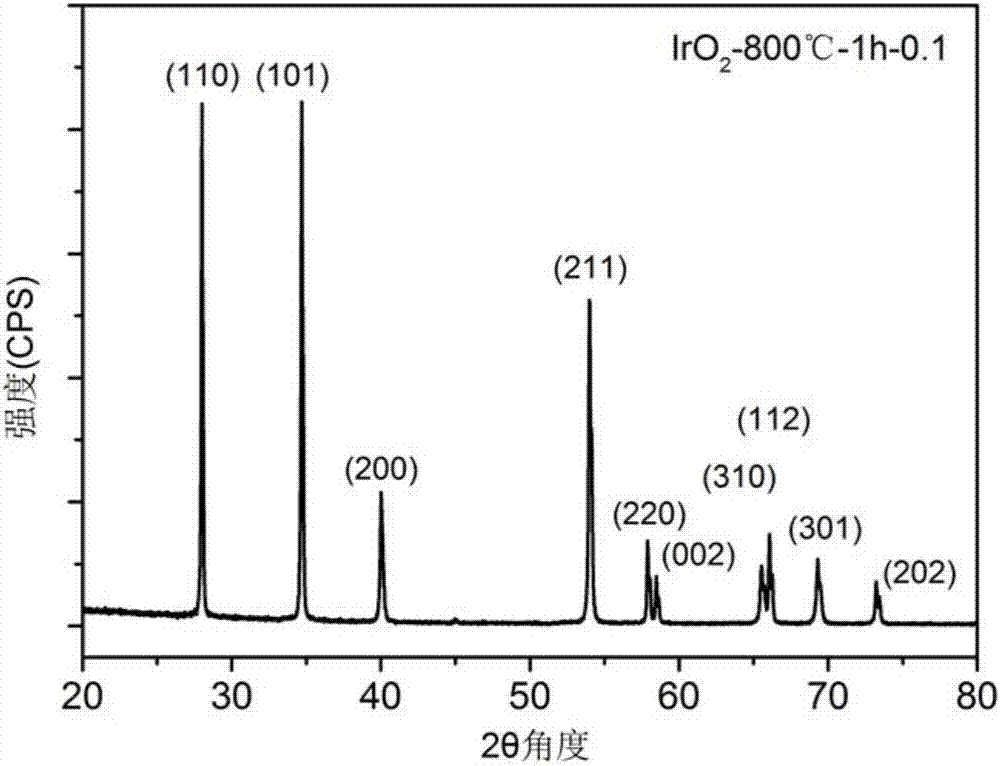
[0031] This embodiment is used to illustrate the preparation method of high-purity iridium dioxide. Specifically, the method includes the following steps:
[0032] (1) take iridium elemental powder and copper oxide powder by the mass ratio of Ir:CuO=1:0.1;
[0033] (2) Put the iridium elemental powder and copper oxide powder prepared in the step (1) into a mortar, grind and mix evenly to obtain a mixed powder, and its particle diameter D90 is ~ 10 microns;
[0034] (3) Put the mixed powder obtained in step (2) into a crucible, put the crucible in a box furnace, and calcine at 800° C. for 1 hour under normal pressure and air atmosphere to obtain a calcined powder;
[0035] (4) Put the calcined powder obtained in step (3) into a beaker, add an appropriate amount of dilute nitric acid, wash away the residual copper oxide powder, and obtain IrO 2 precipitation;
[0036] (5) with step (4) gained IrO 2 The precipitate was washed three times with deionized water and dried to obta...
Embodiment 2
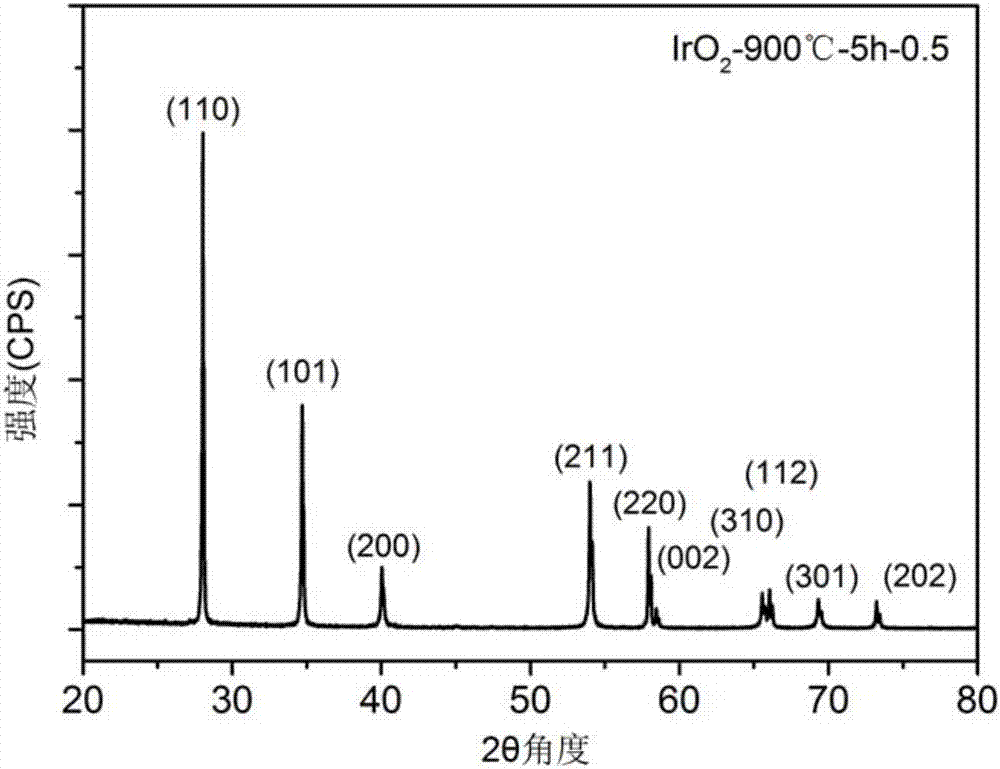
[0040] This embodiment is used to illustrate the preparation method of high-purity iridium dioxide. Specifically, the method includes the following steps:
[0041] (1) take iridium elemental powder and copper oxide powder by the mass ratio of Ir:CuO=1:0.5;
[0042] (2) Put the iridium elemental powder and copper oxide powder prepared in the step (1) into a mortar, grind and mix evenly to obtain a mixed powder, and its particle diameter D90 is ~ 5 microns;
[0043] (3) Put the mixed powder obtained in step (2) into a crucible, put the crucible in a box furnace, and calcine at 900° C. for 5 hours under normal pressure and air atmosphere to obtain a calcined powder;
[0044](4) Put the calcined powder obtained in step (3) into a beaker, add an appropriate amount of dilute nitric acid, wash away the residual copper oxide powder, and obtain IrO 2 precipitation;
[0045] (5) with step (4) gained IrO 2 The precipitate was washed three times with deionized water and dried to obtai...
Embodiment 3
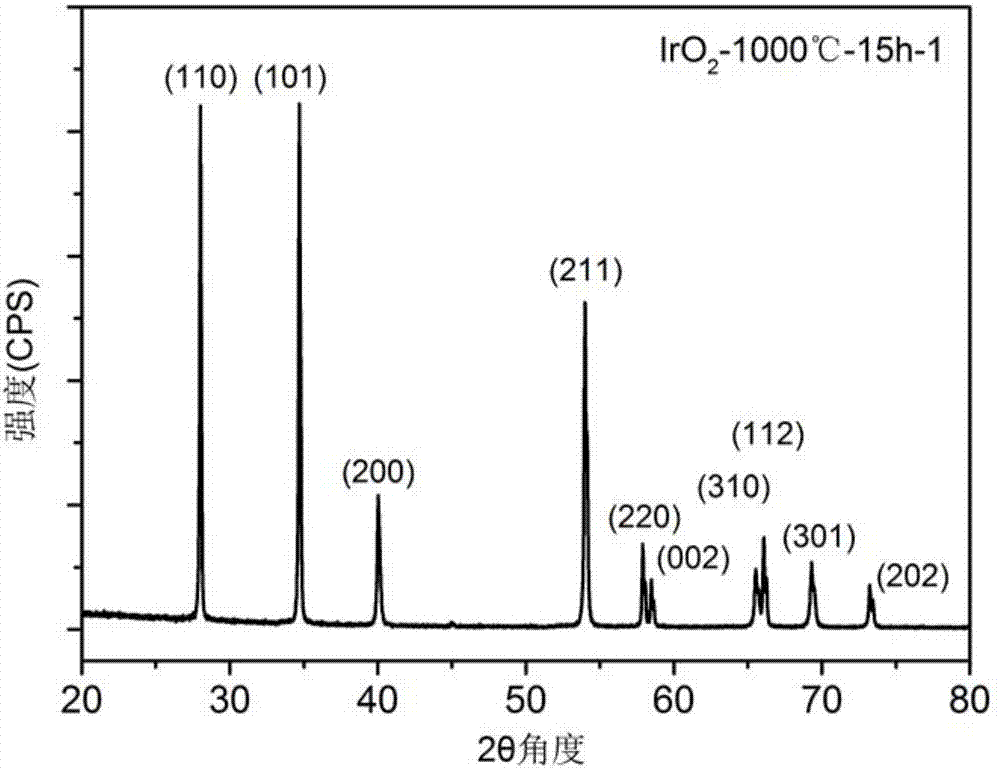
[0049] This embodiment is used to illustrate the preparation method of high-purity iridium dioxide. Specifically, the method includes the following steps:
[0050] (1) take iridium elemental powder and copper oxide powder by the mass ratio of Ir:CuO=1:1;
[0051] (2) Put the iridium elemental powder and copper oxide powder prepared in the step (1) into a mortar, grind and mix evenly to obtain a mixed powder, and its particle diameter D90 is ~ 2 microns;
[0052] (3) Put the mixed powder obtained in step (2) into a crucible, put the crucible in a box furnace, and calcinate at 1000° C. for 15 hours under normal pressure and air atmosphere to obtain a calcined powder;
[0053] (4) Put the calcined powder obtained in step (3) into a beaker, add an appropriate amount of dilute nitric acid, wash away the residual copper oxide powder, and obtain IrO 2 precipitation;
[0054] (5) with step (4) gained IrO 2 The precipitate was washed three times with deionized water and dried to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com