Efficient soluble expression and purification method of Abeta42 in escherichia coli
An Escherichia coli, soluble technology, applied in the fields of biotechnology and genetic engineering, can solve problems such as reducing the efficiency of enzymatic cleavage, and achieve the effects of low cost, large expression amount and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
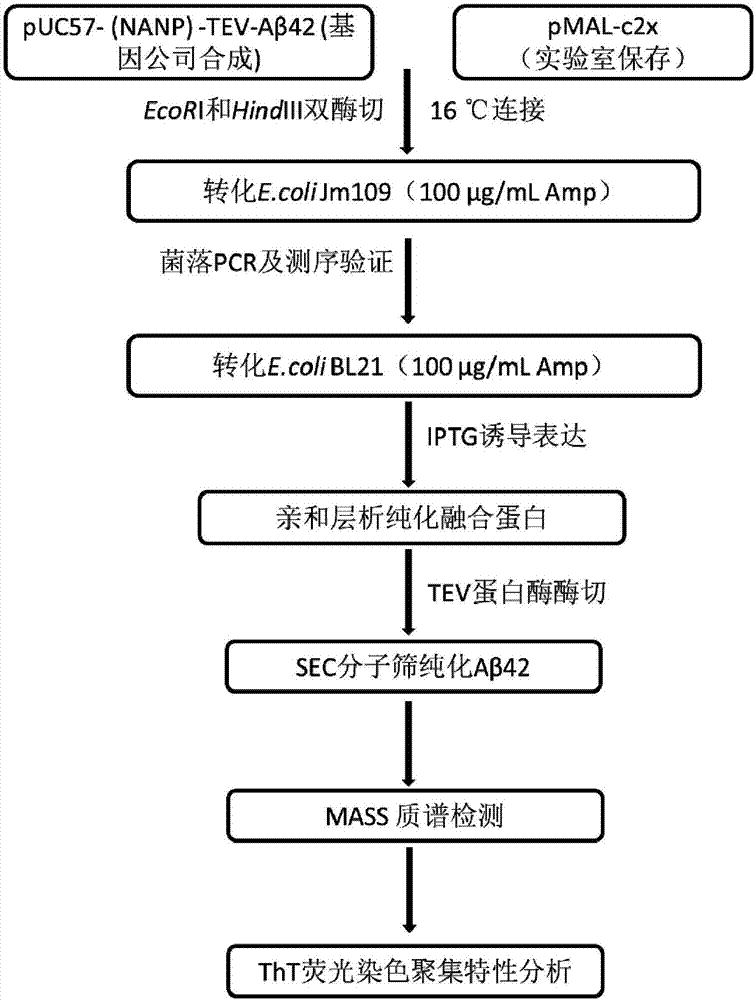
[0060] Example 1: Construction of pMAL-Aβ42 expression vector plasmid and Aβ42 Escherichia coli expression strain
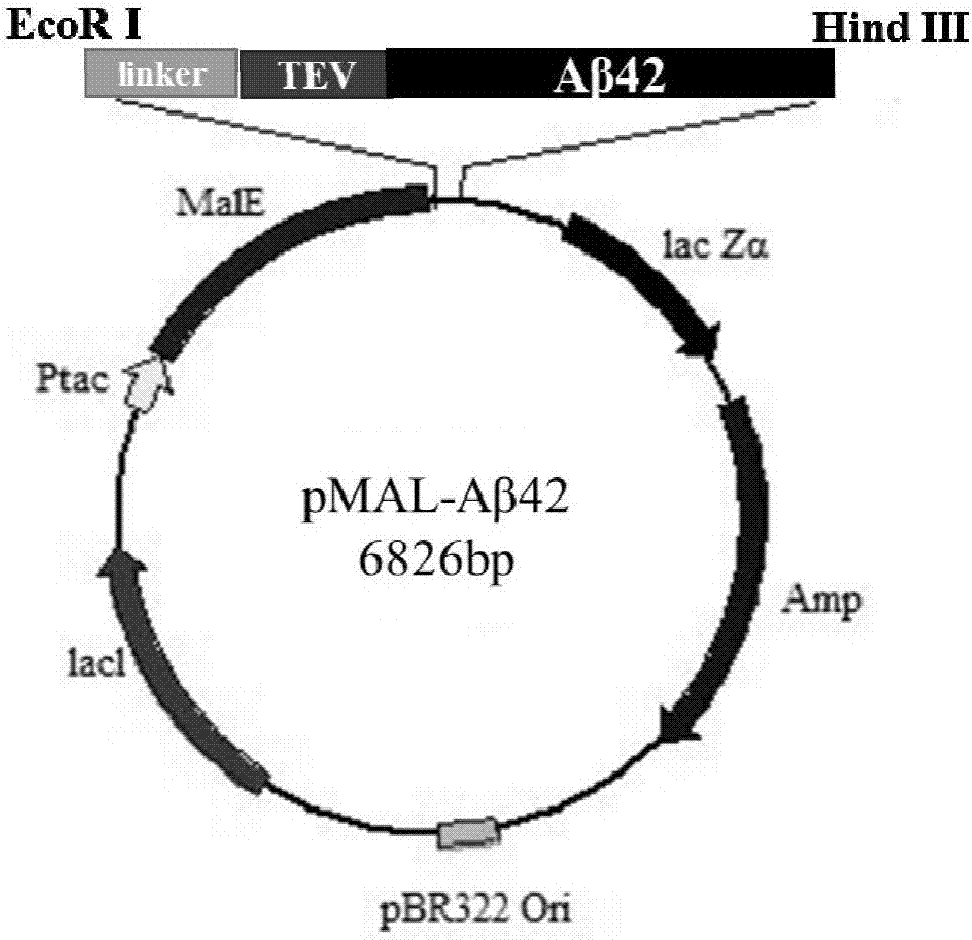
[0061] The schematic diagram of the construction of the expression vector pMAL-Aβ42 is as follows: figure 2 shown;
[0062] (1) First according to (NANP) 3 - The amino acid sequence of TEV-Aβ42 was optimized for expression codon preference in Escherichia coli, and it was synthesized by a gene company (NANP) 3 -TEV-Aβ42 fusion gene fragment, the sequence is as shown in SEQ ID No.1, and the restriction sites at both ends of the target fragment are EcoRI and HindIII respectively;
[0063] (2) with EcoRI and HindIII will (NANP) 3 - The TEV-Aβ42 fragment was excised from the plasmid, and after agarose gel electrophoresis, gel purification was performed to recover the target digestion product. The pMALc2x expression vector plasmid was also double digested with EcoRI and HindIII, and ligated with the target fusion gene fragment obtained above after gel recovery. A...
Embodiment 2
[0068] Example 2: Expression and purification of MBP-Aβ42 fusion protein and purification of Aβ42 polypeptide by proteolysis
[0069] (4) Expression of MBP-Aβ42 fusion protein:
[0070] Pick a single colony of the constructed BL21-MBP-Aβ42 engineering bacteria and culture it overnight at 37°C in 5mL LB medium, transfer it to fresh LB medium according to the inoculum size of 1%, cultivate it at 37°C until the OD600 is 0.6-0.8, add the final The concentration is 0.5mM IPTG induction, the induction temperature is 16°C, and the induction time is 16-18h. Finally, the cells were collected by centrifugation at 6000 rpm for 10 min.
[0071] (5) Purification of MBP-Aβ42 fusion protein:
[0072] Resuspend the bacteria obtained above with lysis buffer (20mM Tris-HCl, pH 7.4, 200mM NaCl, 1mM EDTA, 1mMDTT), add lysozyme and 1% PMSF at a final concentration of 30μg / mL, and ultrasonically break after 30min in ice bath. Centrifuge at 12000rpm for 40min, and collect the supernatant. Add th...
Embodiment 3
[0078] Example 3: Aβ42 polypeptide identification
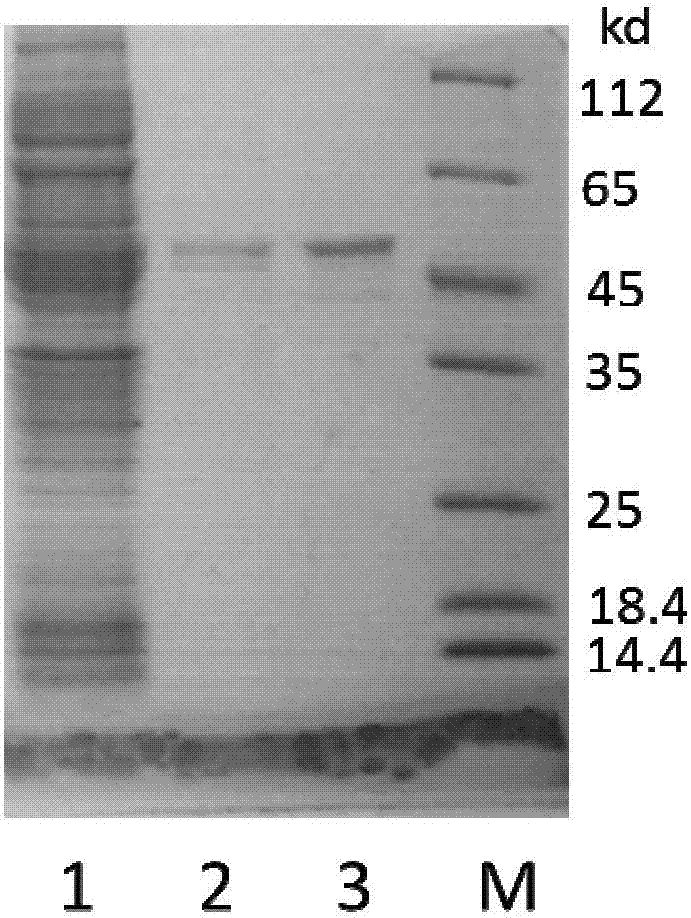
[0079] (1) Electrophoretic identification: the purity and molecular weight of the finally obtained Aβ42 polypeptide were determined by Tricine-SDS-PAGE electrophoresis, as shown in Figure 5 As shown, there is a distinct band around 4.5kDa. The protein concentration was measured by the BCA kit, and about 20mg of Aβ42 polypeptide could be obtained in 1L of bacterial liquid, which was much higher than the existing methods for expressing Aβ42 in other organisms.
[0080] (2) Mass spectrometry identification: the purified Aβ42 polypeptide was subjected to mass spectrometry to identify the molecular weight, such as Figure 6 As shown, the molecular weight is 4539.15, which is consistent with the molecular weight of human amyloid polypeptide Aβ42.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com