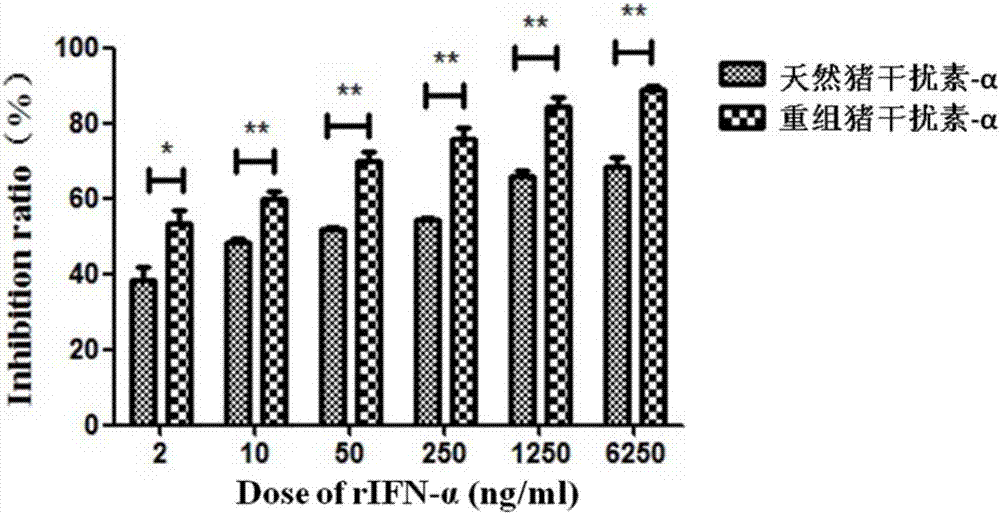
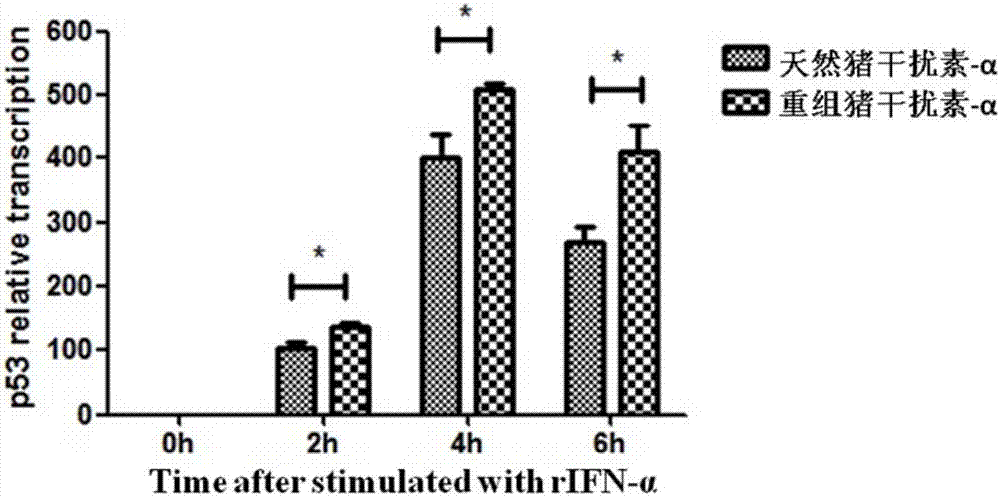
Method for improving antiviral activity of recombinant swine interferon-alpha fusion protein
A porcine interferon and recombinant protein technology, applied in the field of interferon genetic engineering, can solve the problems of inability to guarantee the effective activity of drugs, high cost of use by farmers, and increased production costs, and achieve increased stability and activity, short production time, and easy production. The effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Construction of Recombinant Porcine Interferon-α Gene Plasmid
[0033] 1. Synthesize the required recombinant porcine interferon-α gene by chemical synthesis.
[0034] 2. Construction of recombinant porcine interferon-α gene and pET27b vector
[0035] The recombinant porcine interferon-α gene synthesized in the above step 1 was excised with restriction endonucleases BamHI and HindIII, connected with the pET27b vector cut by the same enzyme and transformed into Escherichia coli DH5ɑ competent, and ampicillin-resistant The recombinant porcine interferon-α gene was cloned into the pET27b vector after plasmid extraction, enzyme digestion identification, and sequencing, and the obtained recombinant plasmid was named pET-rIFN-α.
Embodiment 2
[0036] Example 2 Escherichia coli strain highly expressing recombinant porcine interferon-α gene E. coli Construction of Rosetta / pET-rIFN
[0037] Convert pET-rIFN-α to E. coli Rosetta, transformants were screened on LB plates containing ampicillin, and the obtained recombinants were proved by plasmid extraction, enzyme digestion identification, and sequencing analysis E. coli Rosetta / pET-rIFN-α was as expected.
Embodiment 3
[0038] Embodiment 3 Utilize Escherichia coli engineering bacteria E. coli Production of Recombinant Porcine Interferon-α by Rosetta / pET-rIFN-α
[0039] 1. Culture and fermentation of strains
[0040] Pick Escherichia coli Engineering Bacteria E. coli Rosetta / pET-rIFN-α, the engineered bacteria were inoculated in LB liquid medium according to the inoculum amount of 1%, cultured at a constant temperature of 37°C for 12-14 h, expanded at 1:100 the next day to an OD value of 0.4, and added IPTG to the final Concentration of 0.5mM, continue to cultivate for 3h, 4000r / min, centrifuge for 30min to collect bacteria.
[0041]2. Purification of recombinant porcine interferon-α
[0042] Ultrasonic crushing and centrifugation of the above-mentioned collected bacteria collected the supernatant, filtered the supernatant and purified it using the AKTA protein purification system, followed by affinity chromatography and molecular sieve chromatography to obtain purified recombinant porcin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com