Kit for detecting content of Dabigatran based on thrombin chromogenic substrate method
A chromogenic substrate, dabigatran technology, applied in the field of medical testing, can solve problems such as difficult quantification, weak correlation coefficient, and many interference factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Tris buffer with a concentration of 20mmol / L, then add hydrochloric acid to adjust the pH value to 7.5; then add sodium chloride, polyethylene glycol-8000 calf serum and sodium azide, and stir to obtain the final concentrations of sodium chloride 0.2 mol / L, polyethylene glycol-80000.1%, calf serum 0.1%, sodium azide 0.5mg / ml diluent.
[0043] Dissolve thrombin in diluent to form thrombin solutions with working concentrations of 0.1U / ml, 0.2U / ml, 0.5U / ml, 1U / ml and 2U / ml.
[0044] Take the chromogenic substrate H-D-Phe-Pip-Arg-pNA·2HCl of thrombin and dissolve it in the diluent to form a working concentration of 0.05mmol / L, 0.1mmol / L, 0.3mmol / L, 0.5mmol / L and 1mmol / L of thrombin chromogenic substrate solution.
Embodiment 2
[0046] Sample testing:
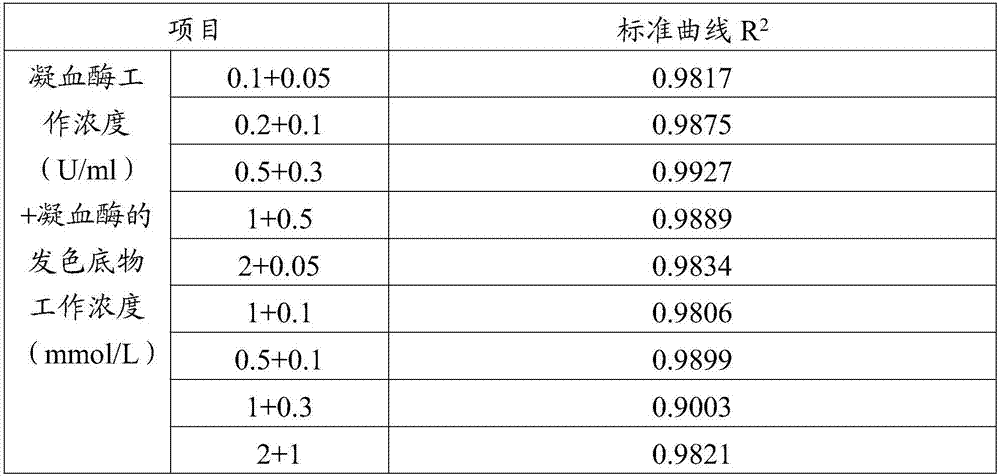
[0047] Take 100 μL sample and mix with 100 μl thrombin solution, incubate at 37°C for 2 minutes, then add 200 μl thrombin chromogenic substrate solution, react at 37°C and measure absorbance at 405nm wavelength for 5 minutes. The selection of the working concentration of the thrombin solution and the chromogenic substrate solution of thrombin is shown in Table 2. Record the test results, calculate the signal intensity, and compare with the standard curve to obtain the dabigatran content.
[0048] Table 1 detects the results of dabigatran assay
[0049]
[0050] It can be seen from Table 1 that the above-mentioned schemes can accurately determine the content change of dabigatran, wherein the working concentration of thrombin is 0.5U / ml and the working concentration of the chromogenic substrate of thrombin is 0.3mmol / L. Detection works best.
Embodiment 3
[0052] Preparation of Dabigatran Standards: Prepare several different concentrations of human plasma standards with known dabigatran concentrations, the concentrations are 0ng / ml, 15ng / ml, 30ng / ml, 60ng / ml, 120ng / ml ml, 250ng / ml, 500ng / ml.
[0053] Standard curve creation:
[0054] (1) Take 100 μL standard solution and 100 μL thrombin solution, mix evenly, and incubate for 2 minutes, the incubation and reaction temperature are both 37°C.
[0055] (2) Then add 200 μL thrombin chromogenic substrate solution, mix well, and measure the absorbance at a wavelength of 405 nm for 5 minutes.
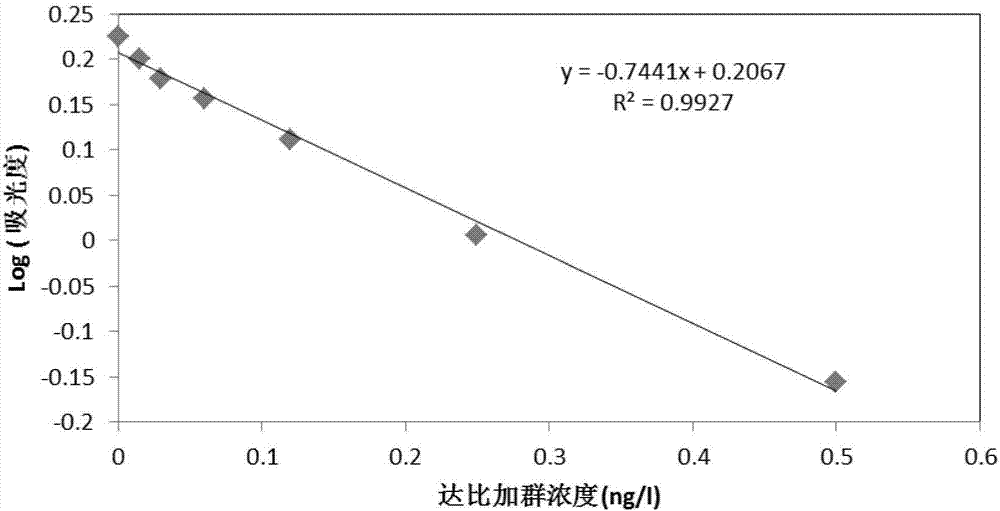
[0056] (3) According to the gradient concentration of dabigatran standard solution and the corresponding absorbance value, a linear equation is used to draw a standard curve, see figure 1 , the standard curve formula is y=-0.7441x+0.2067(R 2 = 0.9927).
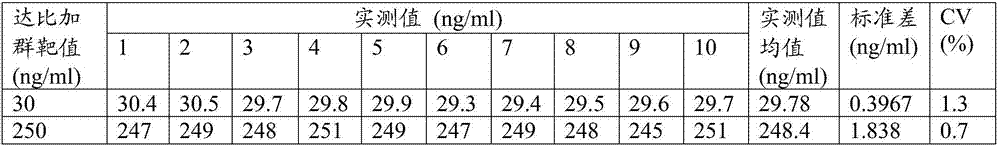
[0057] Table 2 detects the linear range of the dabigatran assay kit
[0058] Dabigatran concentration (ng / ml)
[0059] It can be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com