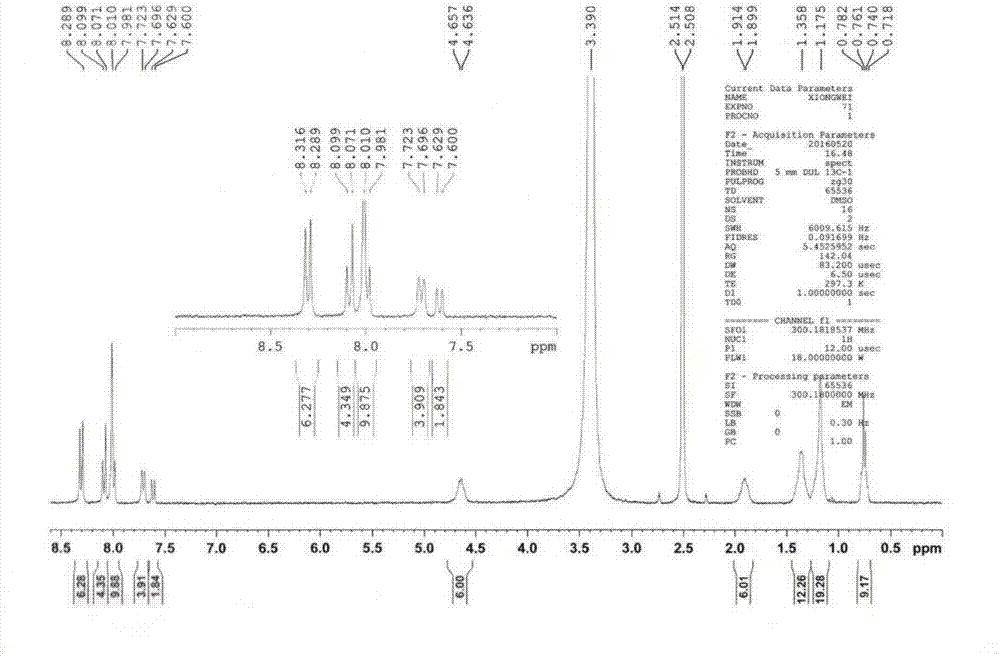
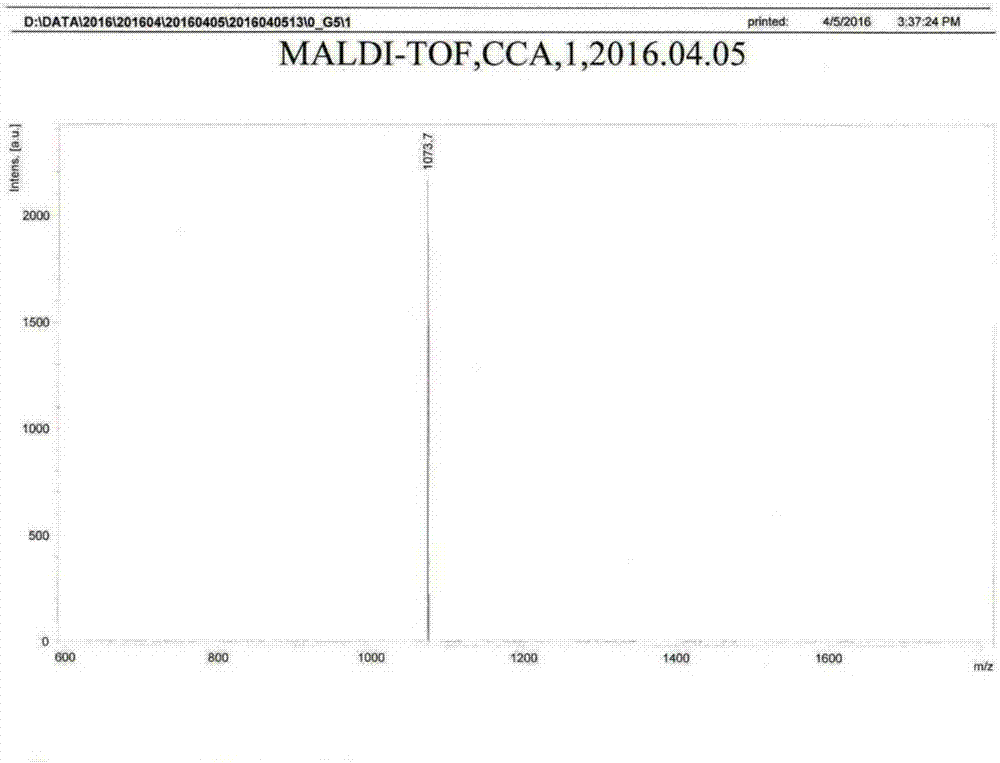
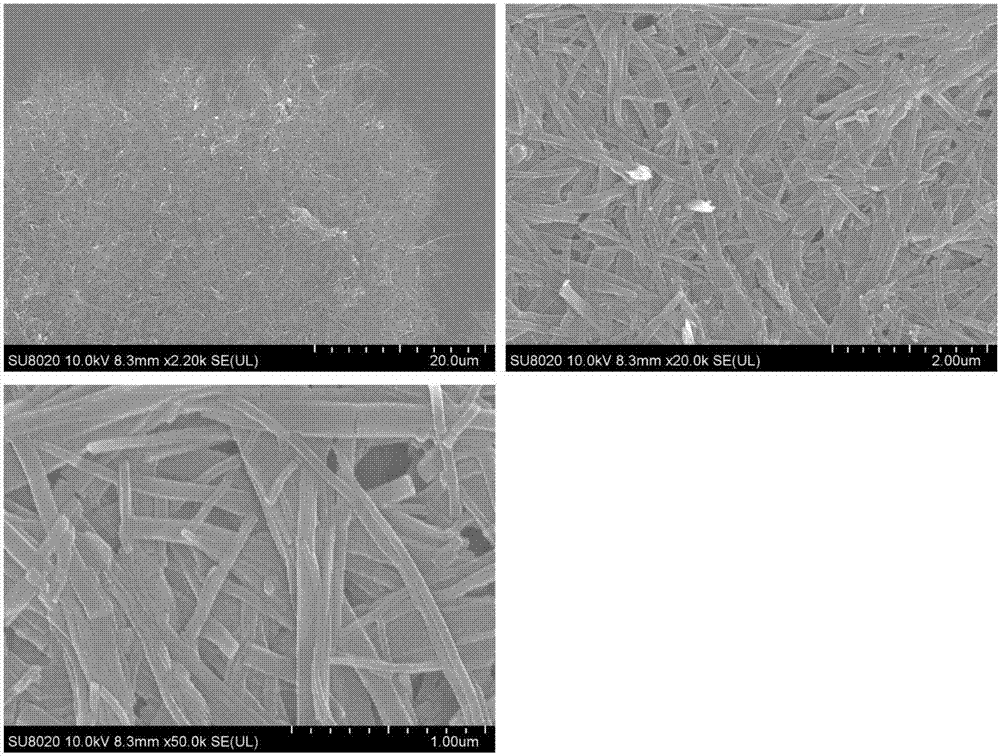
Organic fluorescence sensing composite material with ultrahigh sensitivity response for explosives as well as preparation and application thereof
A technology of fluorescence sensing and composite materials, applied in the field of fluorescence sensing materials, can solve the problems of difficult detection and lack, and achieve the effect of improving detection limit and high fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] As an example, the present invention provides the preparation method of the carbazole derivative when n=3 in the compound shown in (I), and described step (1') specifically comprises:
[0085] In a preferred embodiment of the present invention, the carbazole derivative of n=3 in the preparation formula (I), described step (1 ') specifically comprises:
[0086] (1a). Compound shown in formula (IIa) reacts with RX' to prepare compound shown in formula (IIIa)
[0087]
[0088] X in formula (IIa) and formula (IIIa) is the same or different, and is independently selected from halogen (such as Br, I); X' in RX' is selected from halogen (such as Br, I); Formula (IIIa) and The definition of R in RX' is the same as formula (I);
[0089] (1b). Compound shown in formula (IIIa) and R'B (OH) 2 Reaction makes the compound shown in formula (IV ')
[0090]
[0091] Formula (IV') and R'B(OH) 2 Among them, the definition of R' is the same as formula (I); In formula (IV'), the d...
Embodiment 1
[0138] Prepare compound 1 (in the formula (I), R is a linear octyl group, R' is 4-methoxyacylphenyl, n is 3) with the following structure, and its preparation method is as follows:
[0139]
[0140] (1) Dissolve 1 g of 2,7-dibromocarbazole in 30 ml of N,N-dimethylformamide (DMF) solution, place the above solution in an ice bath at 0°C, and slowly add 1.2 equivalents of 74mg of sodium hydride solid, after continuous stirring for half an hour, 1.5 equivalents of 1-bromooctane was slowly added, and after reacting at room temperature overnight, the product was obtained by column chromatography.
[0141] (2) Get 500 mg of the product obtained in step (1), add 20 ml of 1,4-dioxane solution, add 5 equivalents of bisvaleryl diboron, 14 equivalents of potassium acetate, 10% equivalents of [1,1 '-bis(diphenylphosphino)ferrocene]palladium dichloride was reacted for 6 hours at 80°C under the protection of argon, and the product (TM-1) was obtained by column chromatography.
[0142] (3...
Embodiment 2
[0145] Weigh 150 mg of compound 1 obtained in Example 1 and dissolve it in 10 ml of tetrahydrofuran (THF) solution, add 3-5 equivalents of KOH solid, add 5 ml of water, heat to 65 ° C and react overnight, add 1M HCl aqueous solution to adjust the pH To 2-3, at this time, a light yellow solid precipitated out, and the product was taken out and dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com