Hyperbranched polyoxyethylene ether and polymerized side-chain hyperbranched polycarboxylic acid
A polyoxyethylene ether and polycarboxylic acid technology, which is applied in the field of side chain hyperbranched polycarboxylic acid and its preparation, can solve the problems of hydrogen bonding, hydrophilic surface activity, limited air entrainment and the like, and can reduce the surface tension. and apparent viscosity, increasing side chain density and improving foaming performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The side-chain hyperbranched polycarboxylic acid uses acrylic acid as a small monomer, sodium methacrylate as a chain transfer agent, ammonium persulfate as an initiator, and a two-arm hyperbranched polyoxygen terminated with a methoxy group. Vinyl ether is a macromonomer, and two-arm hyperbranched polycarboxylic acid is obtained through aqueous solution radical copolymerization.
[0070] Its preparation method is as follows:
[0071] (1) Take 0.1 mol of MPEG with four different molecular weights of 400 (n=8), 600 (n=13), 1000 (n=22) and 2000 (n=45) in four three-necked flasks respectively.
[0072] The general formula of MPEG:
[0073] Put 0.2mol of potassium carbonate into 100ml of dichloromethane, add dropwise a mixture of 0.5mol of thionyl chloride and 30ml of dichloromethane under reflux conditions, and react until complete at 45°C, then rinse and reflux with a small amount of dichloromethane Condensation tube, and filter desalination with sand core funnel, the...
Embodiment 2
[0079] The side-chain hyperbranched polycarboxylic acid uses methyl acrylate and maleic anhydride as small monomers, mercaptoacetic acid as a chain transfer agent, and ammonium persulfate as an initiator through aqueous solution radical copolymerization, and methoxy Four-arm hyperbranched polyoxyethylene ether macromonomers terminated by groups are reacted to obtain four-arm hyperbranched polycarboxylic acids.
[0080] Its preparation method is as follows:
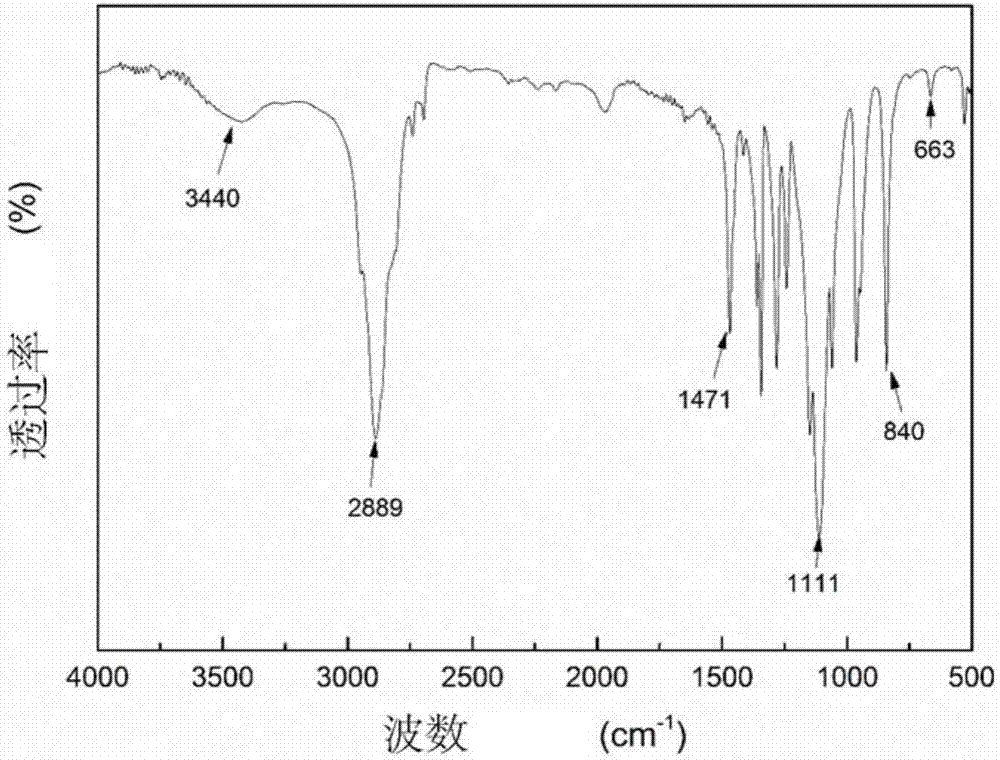
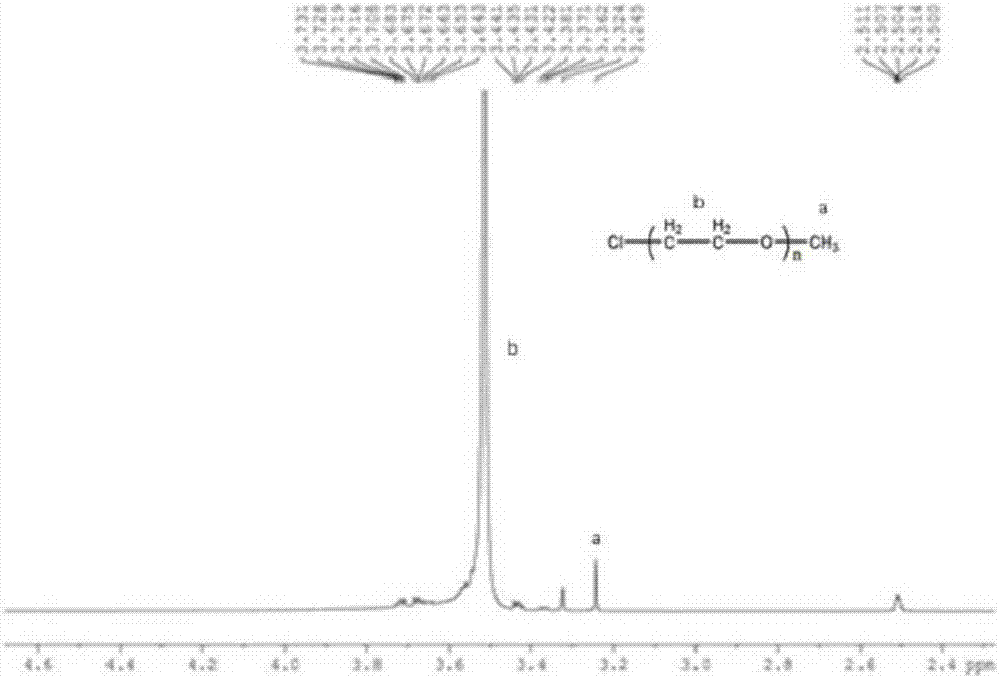
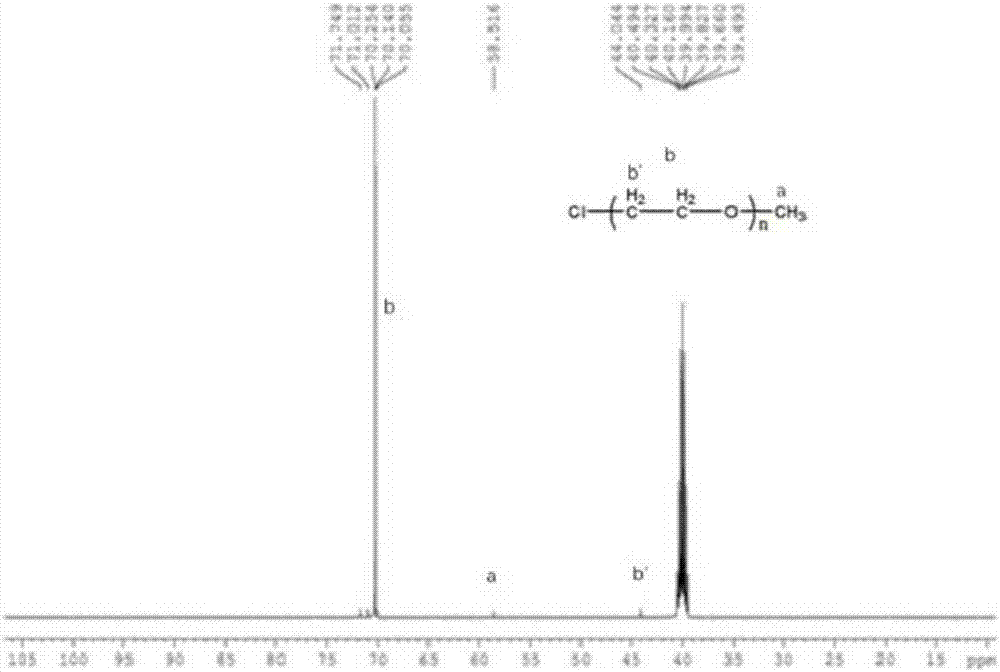
[0081] (1) Take 0.1mol ethanolamine and add 25ml methanol, put it in an ice bath, then add 0.3mol methyl acrylate and add 25ml methanol, put it in a constant pressure dropping funnel, put it under the protection of nitrogen in an ice bath for 0.5h, and then continue to ice bath React for 0.5h, then react in an oil bath at 30°C for 24h in the dark, and distill under reduced pressure at 30°C to obtain M 0.5 ,M 0.5 The FTIR spectrum, 1 H NMR spectrum and 13 C NMR spectrum, see Figure 4~6 . Figure 4 Medium, 3456.36cm ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com