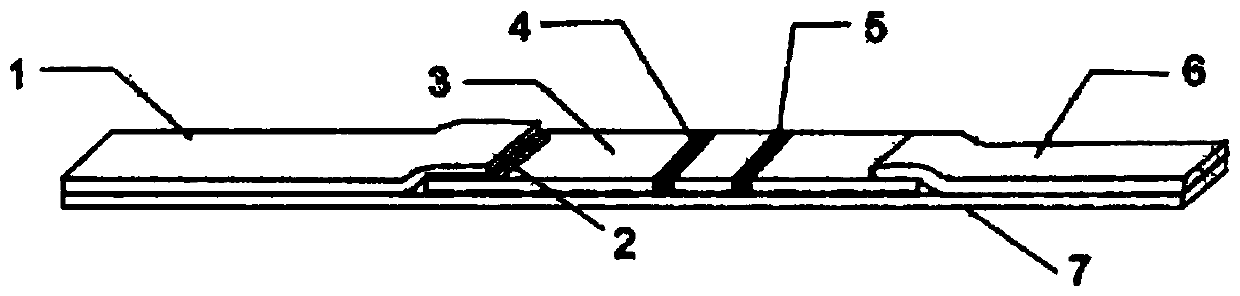
A fluorescent microsphere for labeling specific high-affinity recombinant antibody and its application
A fluorescent microsphere and specific technology, applied in the field of biomedicine, can solve the requirements that immunofluorescence detection reagents are difficult to achieve high sensitivity and high precision, immunofluorescence microparticles have low life activity, and fluorescent microparticle labeling efficiency. Low problems, to achieve accurate and reliable quantitative results, improve reagent sensitivity and linear range, easy and fast operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
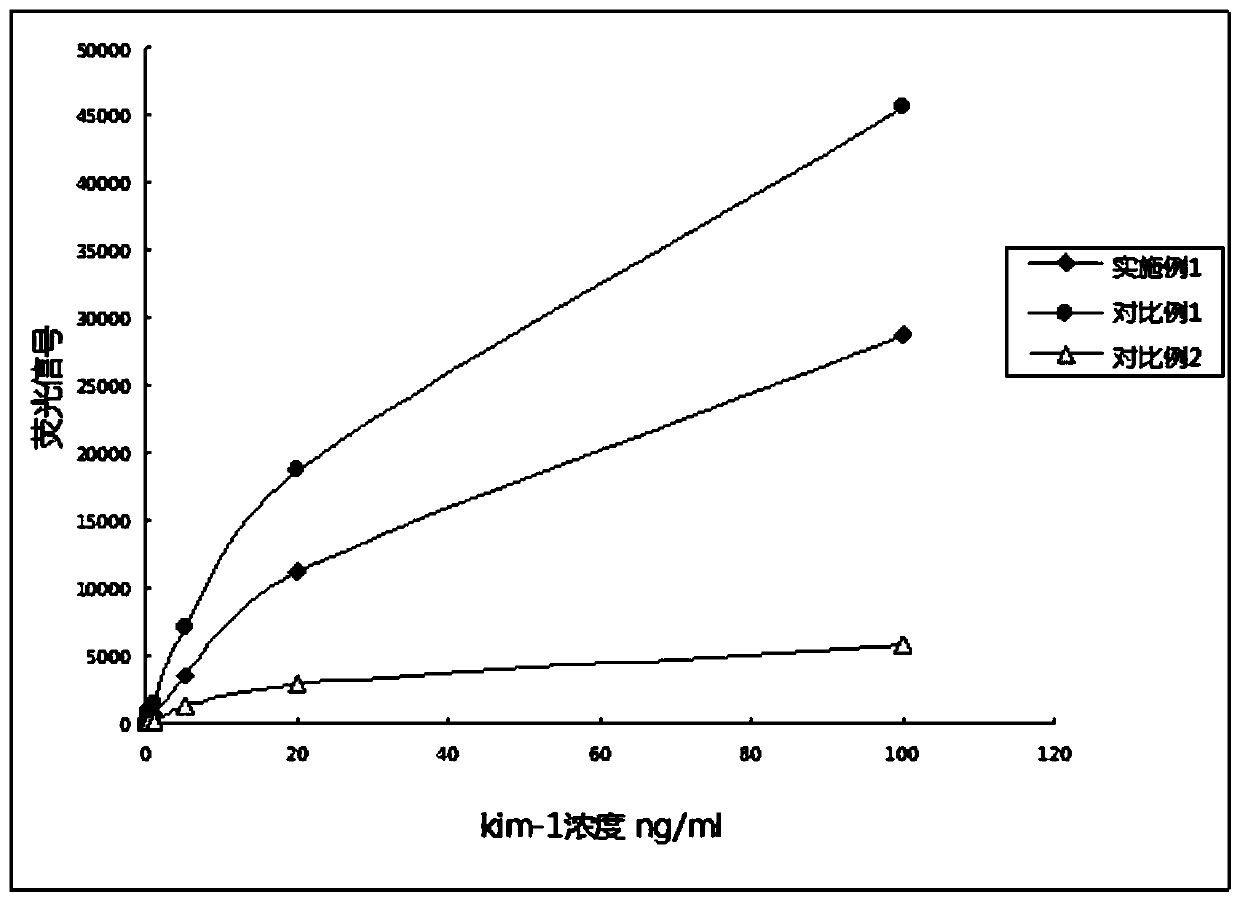
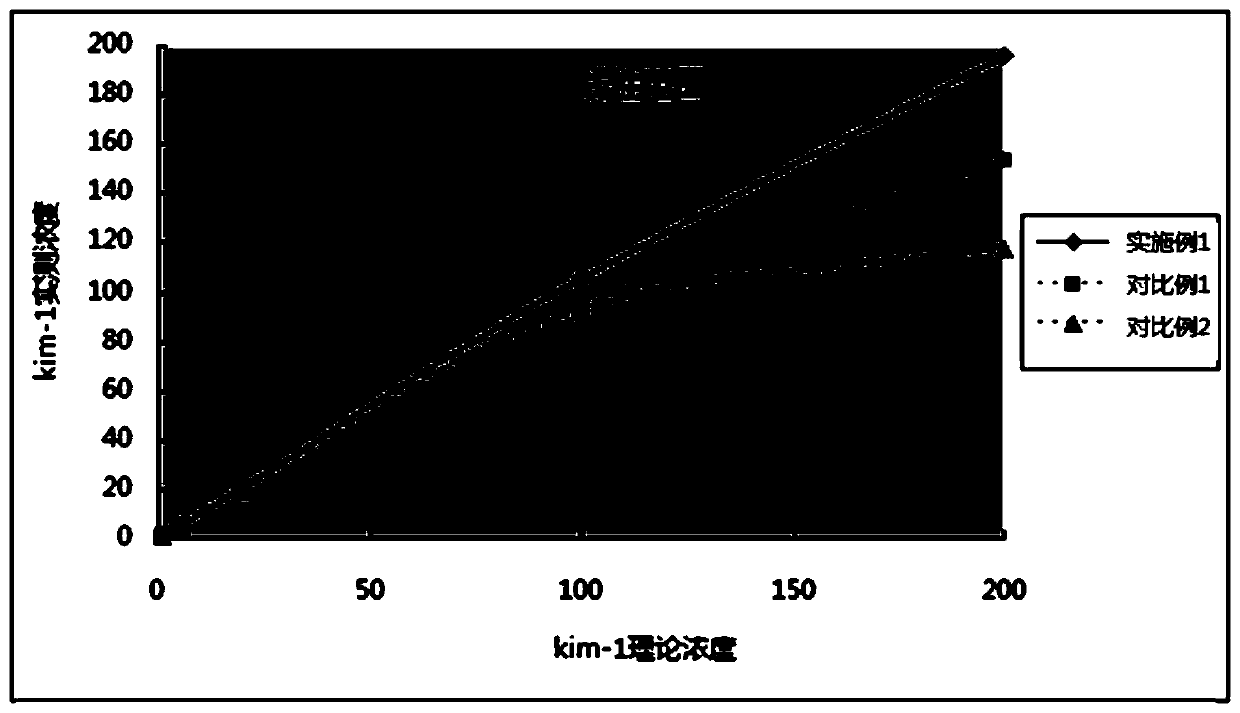
[0071] In Example 1 and Comparative Examples 1 and 2:
[0072] Fluorescent microsphere liquid: particle size of 300nm, solid content of 1%;
[0073] EDC solution: 50mg / ml 1-(3-dimethylaminopropyl)-3 ethylcarbodiimide (EDC);
[0074] NHS solution: 50mg / ml N-hydroxysuccinimide (NHS);
[0075] MES buffer: 0.1M MES PH 5N-morpholine ethanesulfonic acid (MES);
[0076] PBS buffer: 0.05M PBS PH 7.4 phosphate (PBS);
[0077] Amino polyethylene glycol carboxyl (NH 2 -PEG-COOH), the molecular weight is 2000 Dalton.
[0078] Fluorescent microsphere compound solution: containing 0.2wt% BSA, 0.1wt% Trilx X-100, 0.08wt% sodium azide, 0.1M, PH8.0 phosphate buffer.
[0079] Preparation of fluorescence detection card for rapid quantitative detection of KIM-1 in human urine
[0080] 1. Preparation of KIM-1 antibody labeled fluorescent microspheres:
[0081] 1.1 Take 1ml of fluorescent microsphere solution, add 0.1ml of EDC solution, and activate at room temperature for 15 minutes;
[0082] 1.2 Add 0.1ml of NHS...
Embodiment 2
[0181] In Example 2 and Comparative Examples 3 and 4:
[0182] Fluorescent microsphere liquid: particle size of 300nm, solid content of 1%;
[0183] EDC solution: 50mg / ml 1-(3-dimethylaminopropyl)-3 ethylcarbodiimide (EDC);
[0184] NHS solution: 50mg / ml N-hydroxysuccinimide (NHS);
[0185] MES buffer: 0.1M MES PH 5N-morpholine ethanesulfonic acid (MES);
[0186] PBS buffer: 0.05M PBS PH 7.4 phosphate (PBS);
[0187] Amino polyethylene glycol carboxyl (NH 2 -PEG-COOH), the molecular weight is 2000 Dalton.
[0188] Fluorescent microsphere compound solution: containing 0.2wt% BSA, 0.1wt% Trilx X-100, 0.08wt% sodium azide, 0.1M, PH8.0 phosphate buffer.
[0189] Preparation of fluorescence detection card for rapid quantitative detection of NGAL in blood
[0190] 1. Preparation of NGAL antibody labeled fluorescent microspheres:
[0191] 1.1 Take 1ml of fluorescent microsphere solution, add 0.1ml of EDC solution, and activate at room temperature for 15 minutes;
[0192] 1.2 Add 0.1ml of NHS solutio...
Embodiment 3
[0293] In Example 3 and Comparative Examples 5 and 6:
[0294] Fluorescent microsphere liquid: particle size 250nm, solid content 1%;
[0295] EDC solution: 50mg / ml 1-(3-dimethylaminopropyl)-3 ethylcarbodiimide (EDC);
[0296] NHS solution: 50mg / ml N-hydroxysuccinimide (NHS);
[0297] MES buffer: 0.1M MES PH 5N-morpholine ethanesulfonic acid (MES);
[0298] PBS buffer: 0.05M PBS PH 7.4 phosphate (PBS);
[0299] Amino polyethylene glycol carboxyl (NH 2 -PEG-COOH), the molecular weight is 1000 Dalton.
[0300] Fluorescent microsphere compound solution: containing 0.2wt% BSA, 0.1wt% Trilx X-100, 0.08wt% sodium azide, 0.1M, PH8.0 phosphate buffer.
[0301] Preparation of fluorescence detection card for rapid quantitative detection of IGFBP-7 in urine
[0302] 1. Preparation of IGFBP-7 antibody labeled fluorescent microspheres:
[0303] 1.1 Take 1ml of fluorescent microsphere solution, add 0.1ml of EDC solution, and activate at room temperature for 15 minutes;
[0304] 1.2 Add 0.1ml of NHS solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com