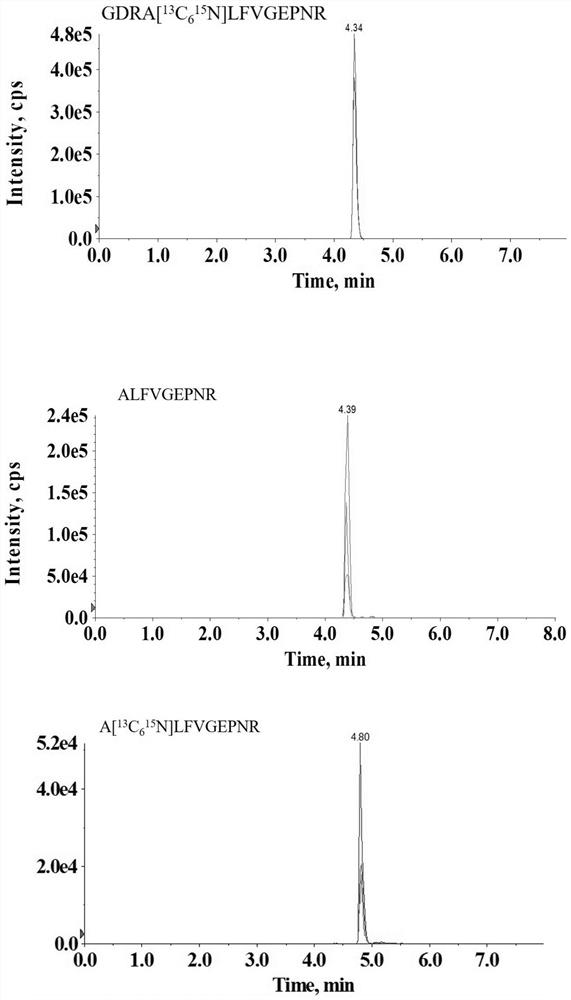
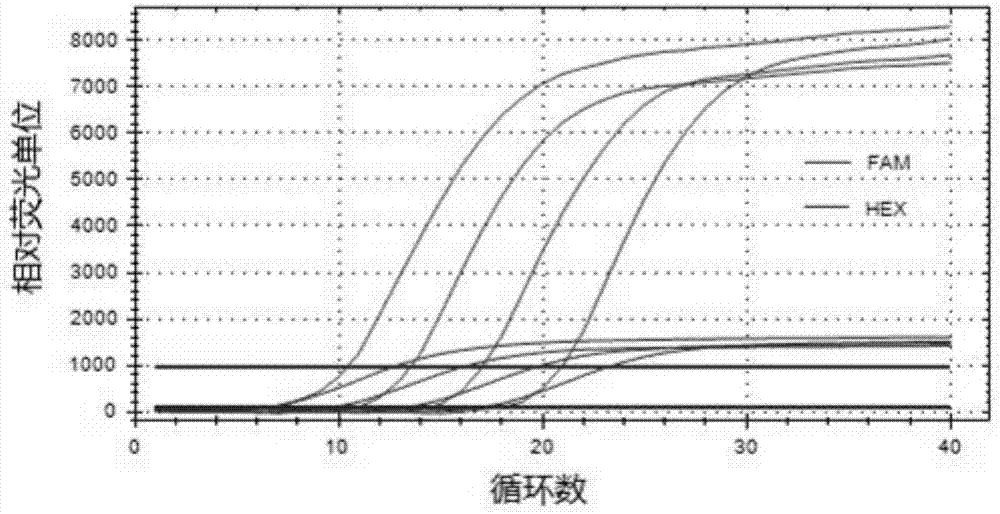
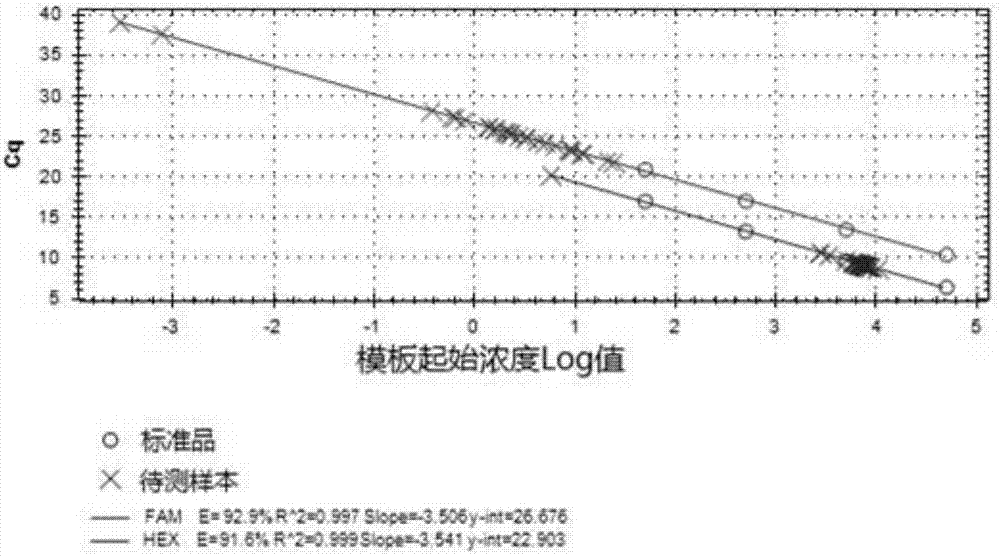
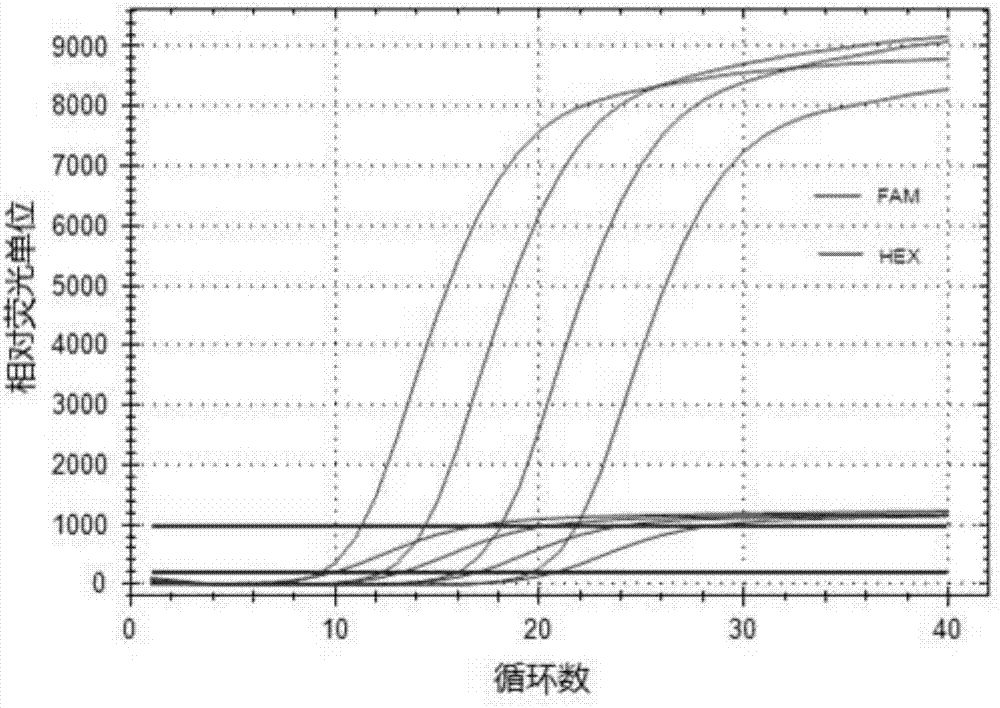
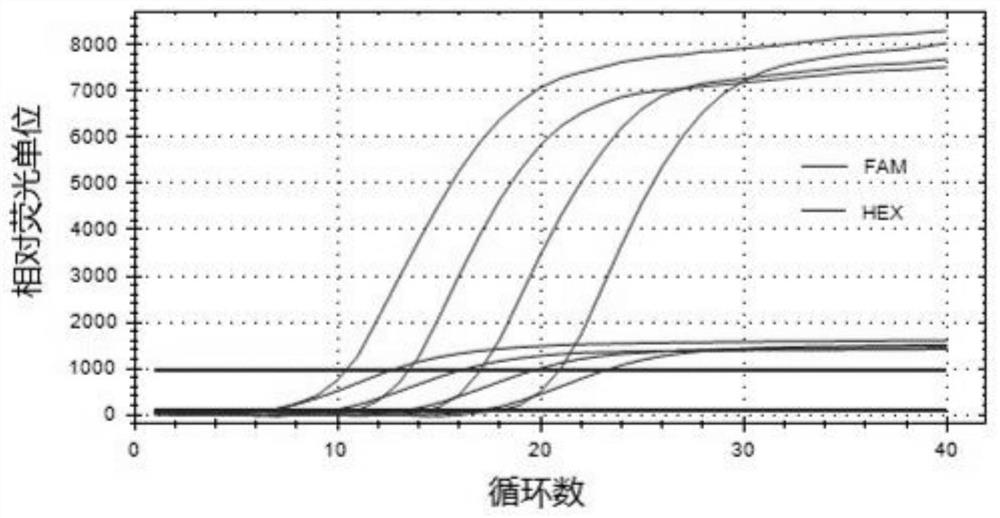
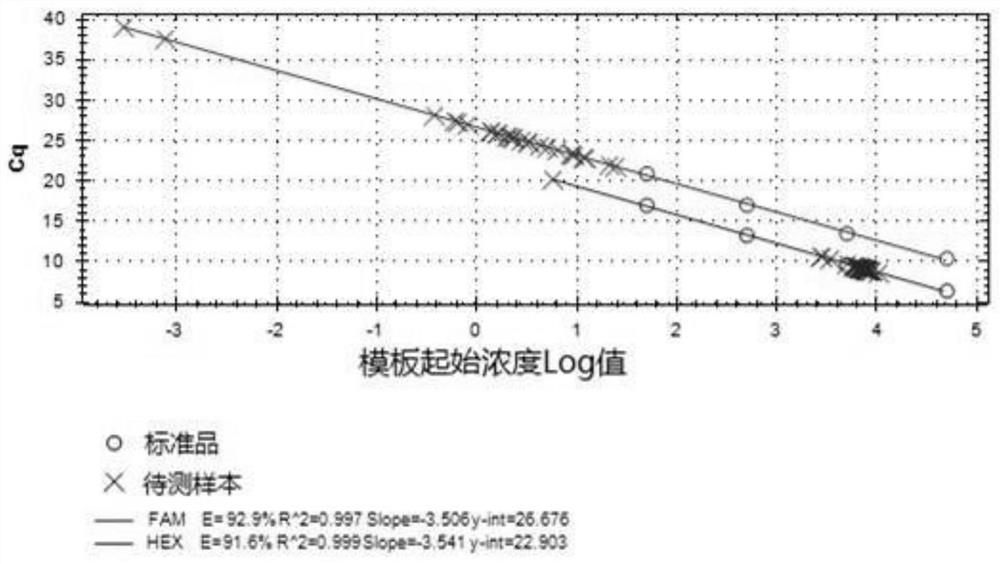
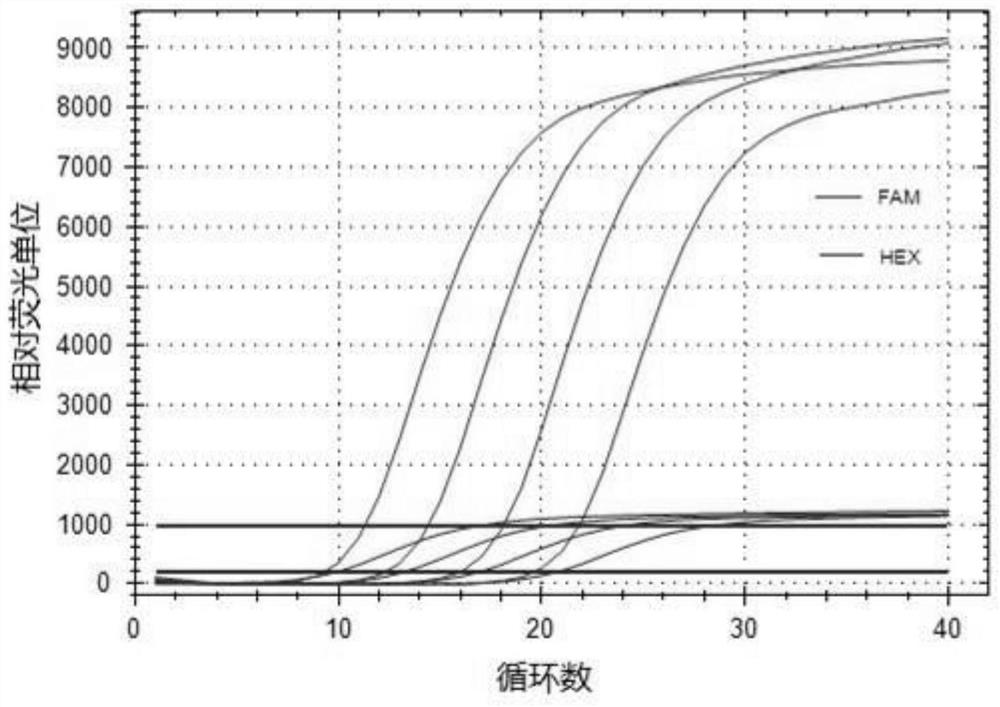
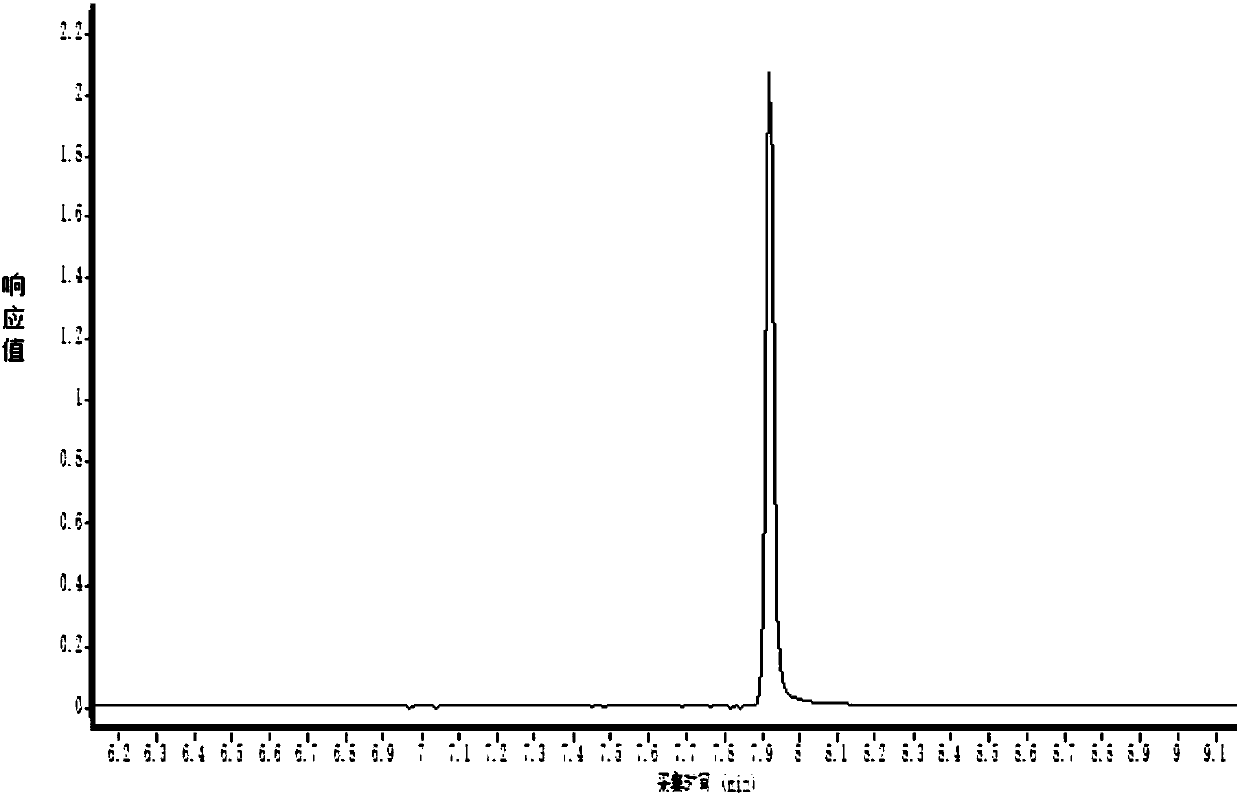
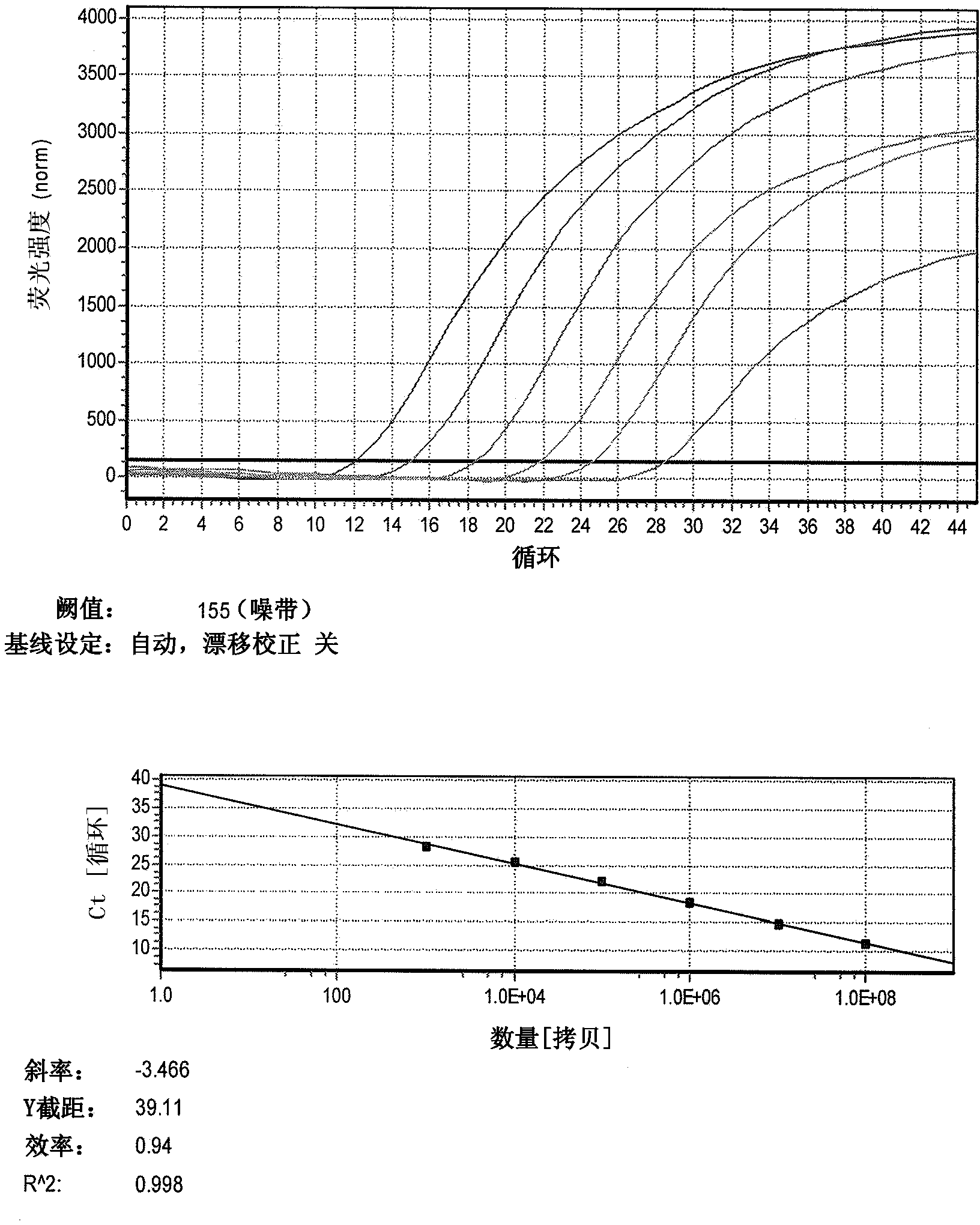
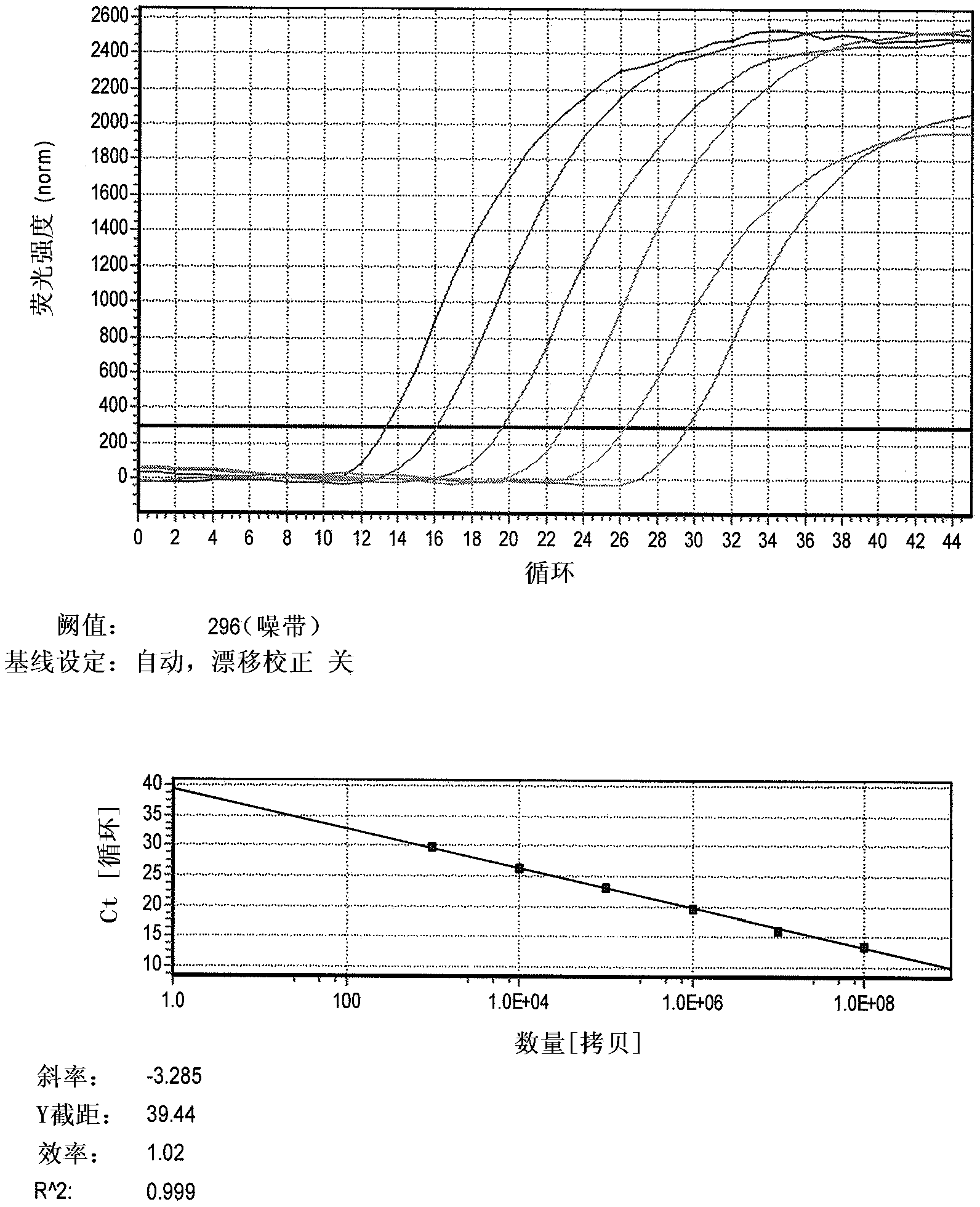
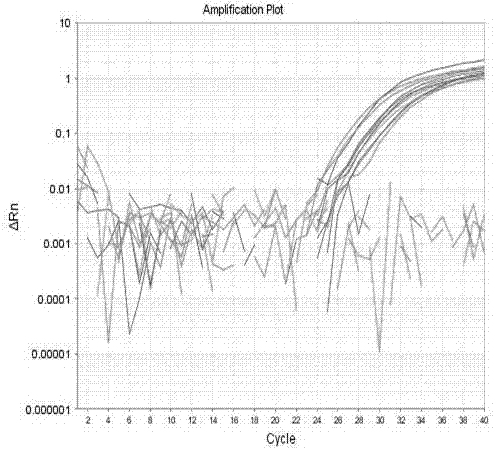
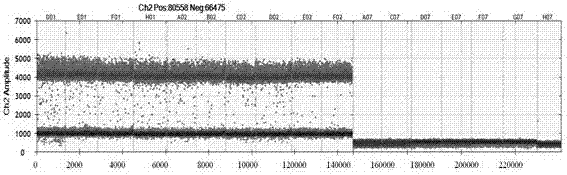
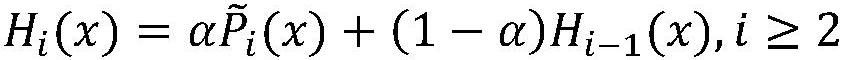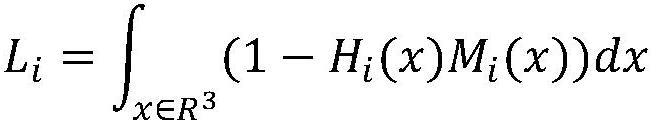Patents
Literature
44results about How to "Quantitative results are accurate and reliable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
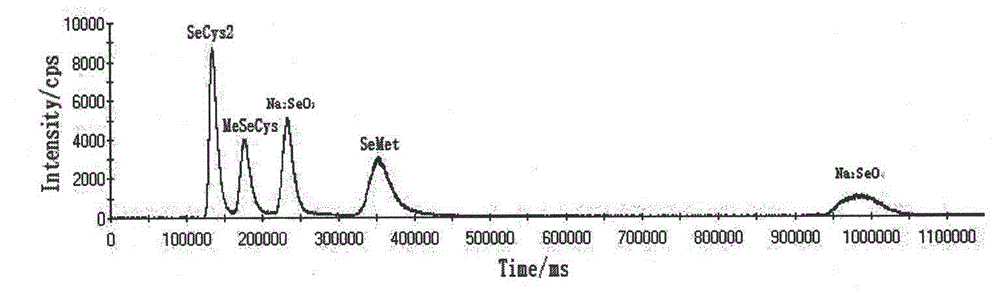
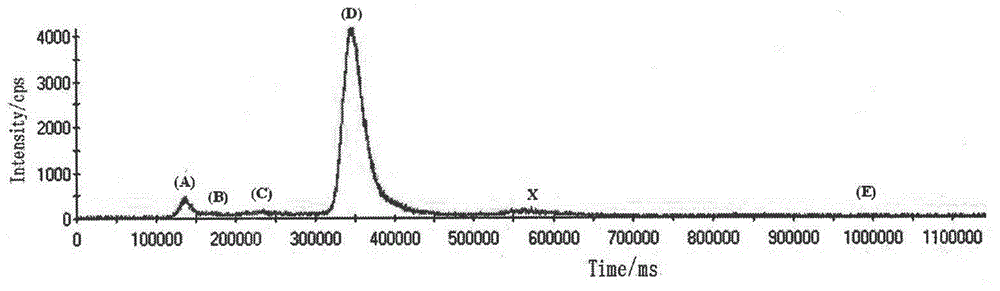
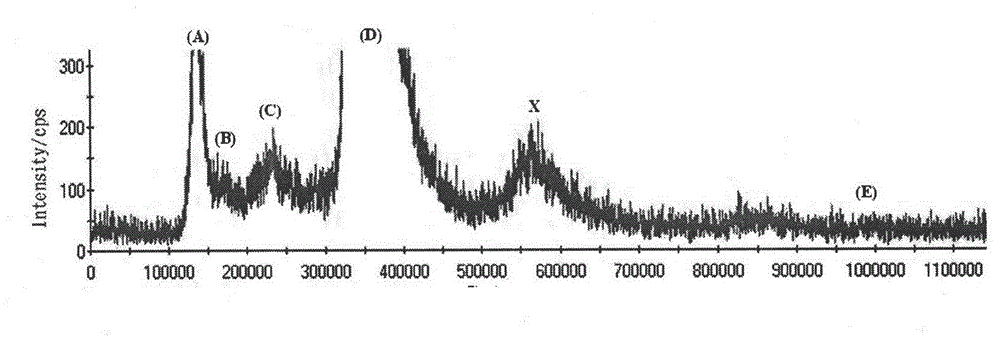
Detection method of selenium forms in plants
InactiveCN102721779ASimple and fast pre-processingSingle mobile phaseComponent separationFreeze-dryingInductively coupled plasma
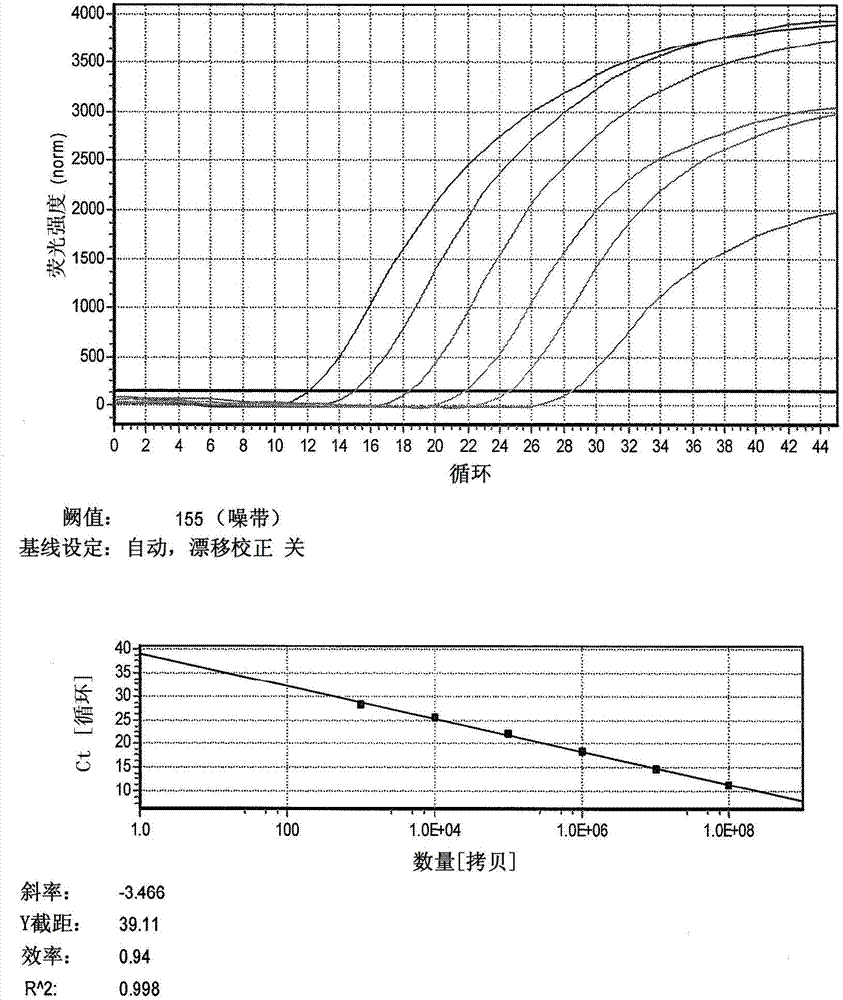
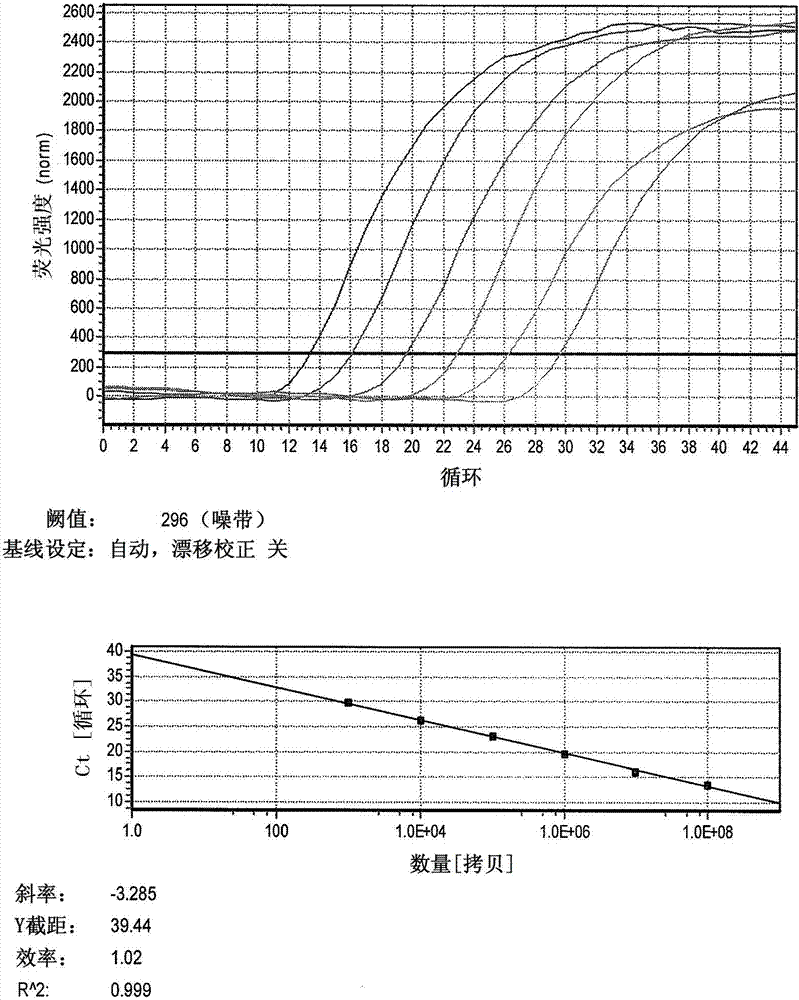
The invention relates to a detection method of selenium forms in plants. The method comprises the steps of: 1, previous preparation of detection: determining instruments and reagents for detection; 2, establishing high performance liquid chromatography and inductively coupled plasma mass spectrometry, that is, HPLC-ICP-MS hyphenated techniques; 3, establishing chromatograms of five selenium form standard solutions; 4, pretreatment of plant samples and extraction and detection of selenium forms: cleaning plant materials to be detected with ultrapure water, carrying out freeze drying, smashing and filtering, measuring the water content, sealing, placing and storing at 4 DEG C for standby; or performing ultrasonic extraction at 37 DEG C for 30 minutes by enzyme immediately, taking supernatant after centrifugalization, detecting the selenium forms according to the HPLC-ICP-MS hyphenated techniques, and calculating the content of selenium forms in plants according to peak areas corresponding to the selenium forms in the samples shown in collected maps. According to the method for detecting the selenium forms in plants, forms are separated completely, pretreatment of samples is simple, convenient and rapid, the moving phase is single in component and simple to prepare, the repeatability and the precision are good, and the quantitative result is accurate and reliable.
Owner:ZHEJIANG FORESTRY UNIVERSITY
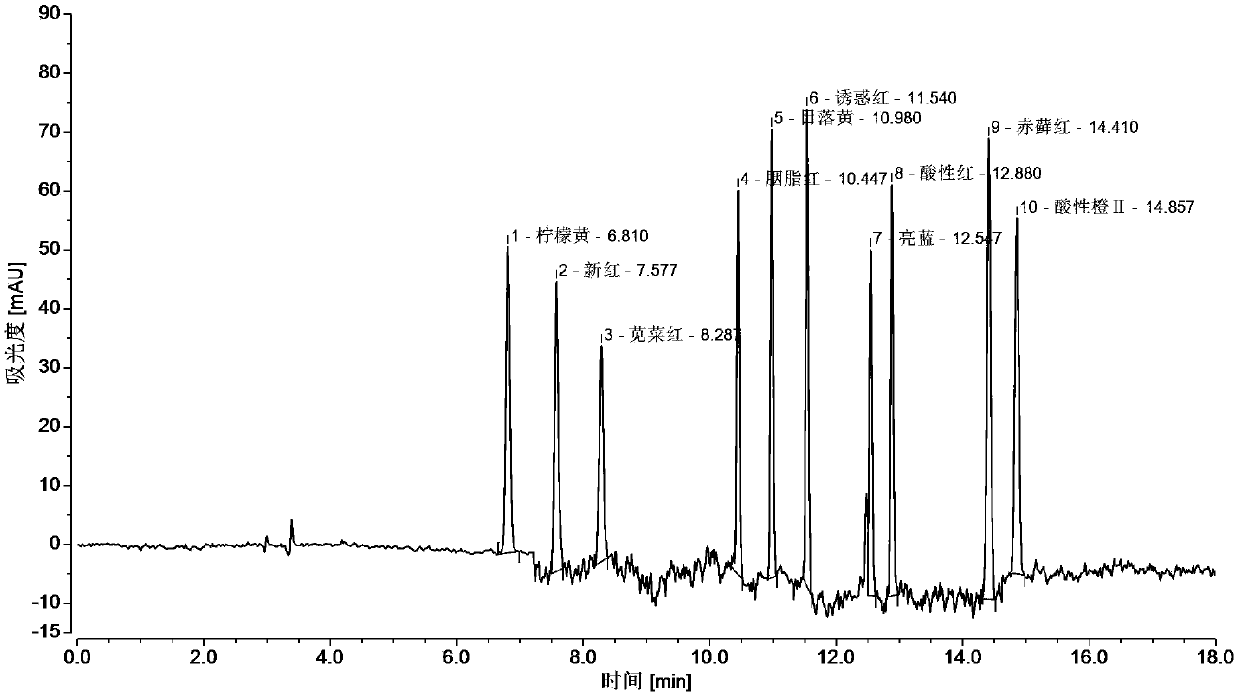
Method of simultaneously detecting various synthetic pigments in wheaten foods via HPLC-DAD
InactiveCN107748210AAvoid interferenceSimplify preprocessing stepsComponent separationHplc dadFood additive
The invention relates to the technical field of detection of synthetic pigments and especially relates to a method of simultaneously detecting various synthetic pigments in wheaten foods via HPLC-DAD.The method includes the following steps: A) preparation of standard work solutions and samples; b) extraction of a sample solution; C) HPLC-DAD analysis to the solution prepared in the step B); D) calculation of content of to-be-detected substances with standard curve. In the HPLC-DAD detection method, pretreatment steps are simplified, wherein solid-phase extraction and concentration steps are avoided, thus reducing pretreatment time. Detection is carried under wavelength of 400-600 nm, so that interference due to majority of food additives and natural pigments is avoided. The method is accurate, quick and simple, has reliable quantitative results, can effectively detect ten artificially synthetic pigments in one time, and is very suitable for simultaneously treating samples in multi-batches.
Owner:昌邑市检验检测中心
Real-time quantification PCR chip used for detecting gene expression of mouse cholesterol metabolism
InactiveCN103820552AHigh amplification efficiencyGood repeatabilityMicrobiological testing/measurementPcr chipTarget analysis
The invention relates to a real-time quantification PCR chip used for detecting gene expression of mouse cholesterol metabolism. The real-time quantification PCR chip uses 88 genes closely related to cholesterol metabolism as detection sites and selects 4 house-keeping genes. The real-time quantification PCR chip provided by the invention can carry out target analysis on gene related to mouse cholesterol metabolism, needs a short experimental period, has simple data processing and low cost, does not need special experiment organization detection, needs only a quantification PCR instrument and has the advantages of high amplification efficiency, reliable quantification results, good repeatability and high specificity.
Owner:DONGHUA UNIV
Single cell real time fluorescent quantitative RT-PCR method for detecting foot-and-mouth disease virus genome RNA
InactiveCN101386893APerfect research methodWide applicabilityMicrobiological testing/measurementFluorescenceNormal cell
The invention discloses a method for detecting real-time fluorescence quantitative RT-PCR of a single cell of foot and mouth disease virus genome RNA. The method utilizes a microinjection instrument to separate single cell of a foot and mouth disease virus; and after cracking, the fluorescence quantitative RT-PCR is used to carry out quantitative analysis. Visible operation of the microinjection instrument can rapidly and accurately separate out the single cell; and the microinjection instrument is combined with the high-sensitivity fluorescence quantitative RT-PCR to realize the detection of the quantity of the virus genome RNA in the single cell. The method can be used for researching virus copying on the level of the single cell and the relation between the single cell and a host cell and provides a new method for in-depth research of virus infection cytobiology. The technology for separating and cracking the cells has wide applicability, can be directly applied to the separating pretreatment of other kinds of virus-infected cells or normal cells in order that the method can be also used for the detection of other RNA virus genomes, the copying of normal cell genomes and the research on a transcribed molecular mechanism.
Owner:广州誉嘉生物科技有限公司
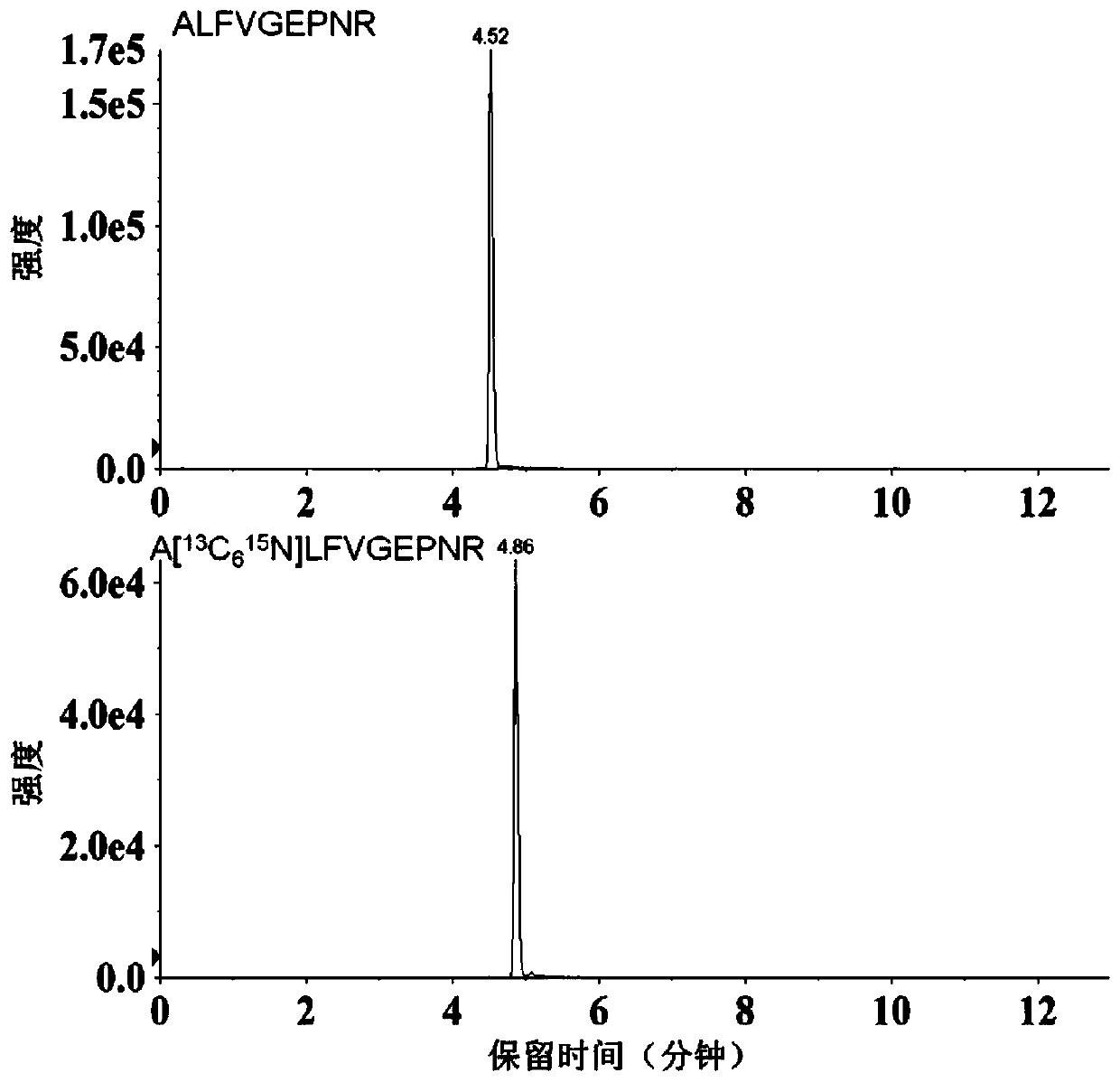
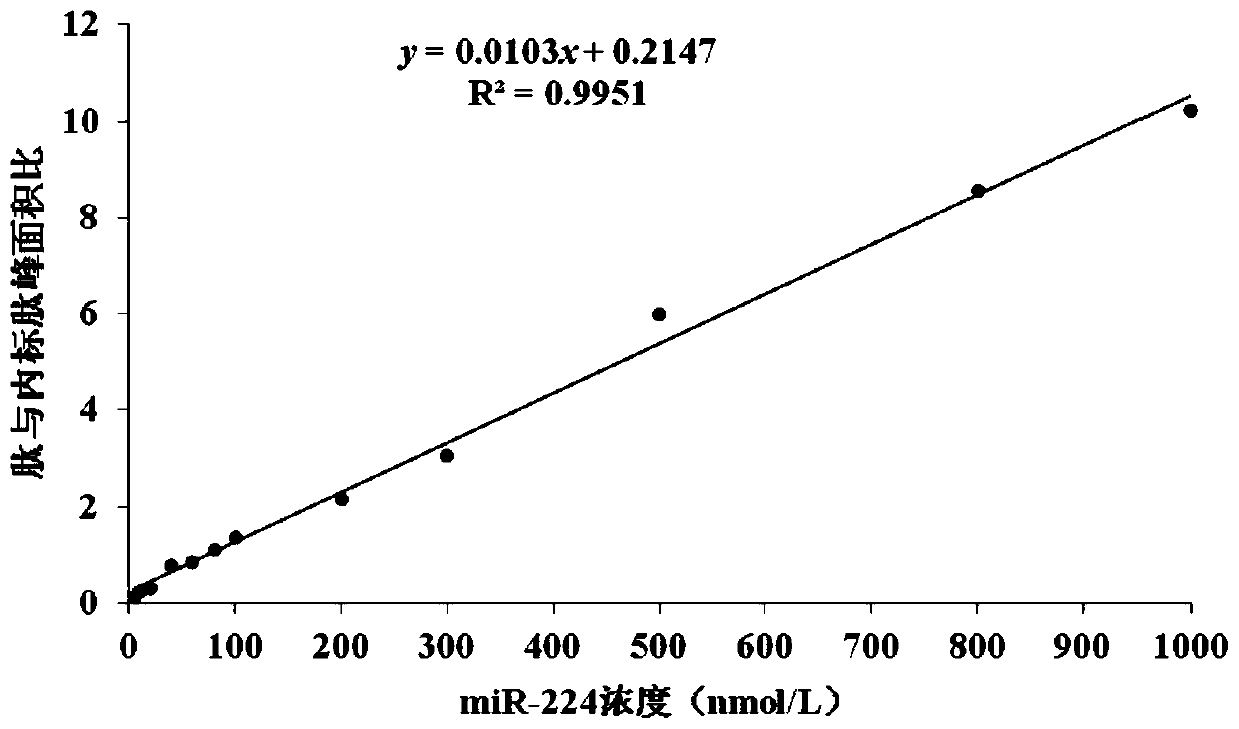
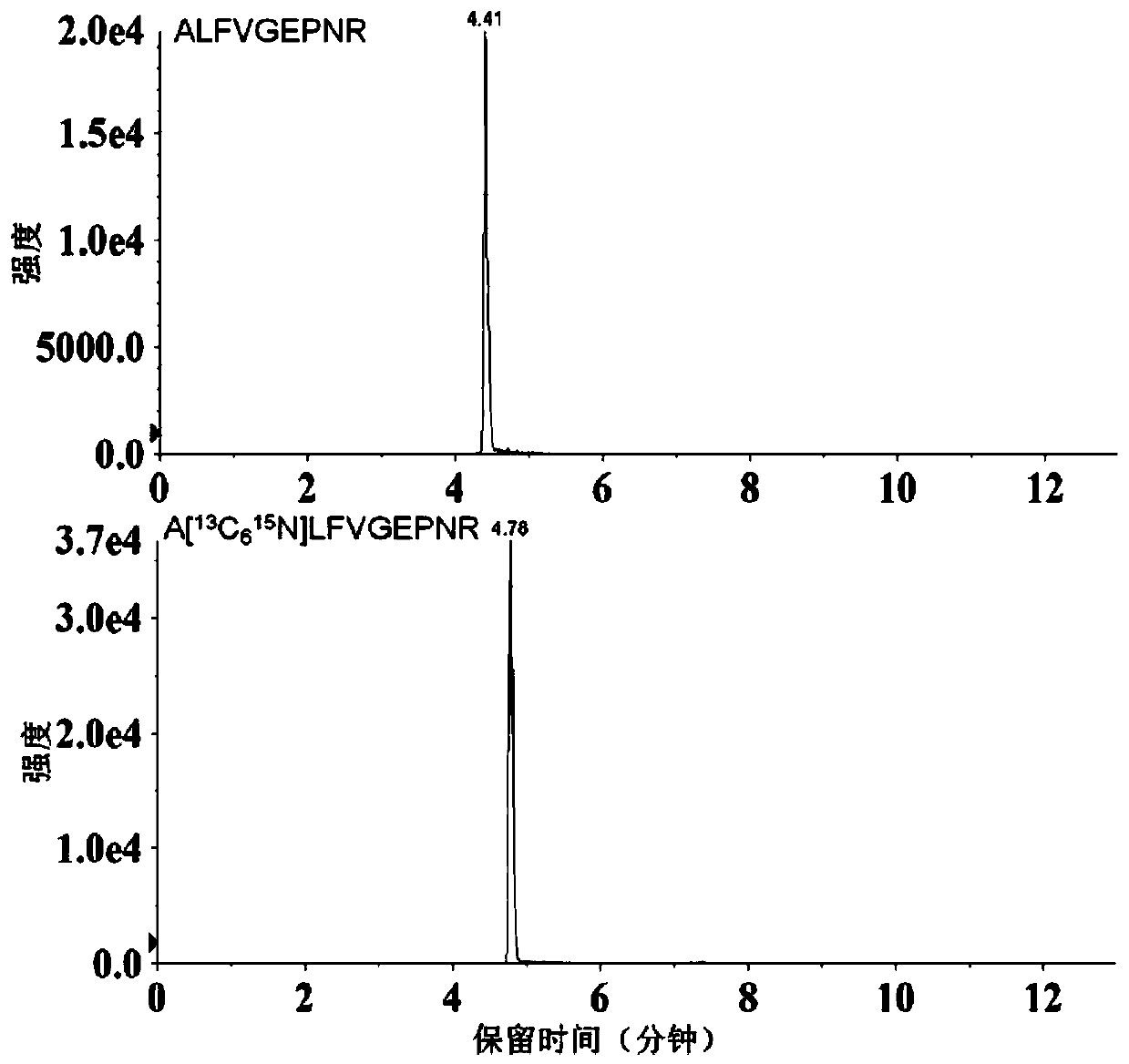
Method for determining serum miR-224 content by using isotope dilution mass spectrometry
ActiveCN110220994ALow detection limitQuantitative results are accurate and reliableComponent separationHydrolysateSolid phase extraction
The present invention discloses a method for determining the serum miR-224 content by using the isotope dilution mass spectrometry. The method comprises the following steps: (1) synthesizing a singlestrand of miR-224 nucleic acid to formulate into a solution, and establishing a standard curve; (2) synthesizing a nucleic acid peptide probe to formulate into the solution for capturing miR-224; (3)synthesizing the isotopically labeled polypeptide to formulate into the solution as an internal standard; (4) extracting the miRNA in a serum sample; (5) biotinylating the extracted miRNA; (6) reacting the miRNA with the streptomycin agarose sphere to form the miRNA-biotin-streptavidin agarose sphere complex; (7) adding the nucleic acid peptide probe to capture the miR-224-biotin-streptavidin agarose sphere complex; (8) after washing the complex, adding trypsin for enzymatic hydrolysis, then performing solid phase extraction on the enzymatic hydrolysate, and drying and re-dissolving the extracted polypeptide; (9)performing mass spectrometry on the dissolved polypeptide and the isotopically labeled polypeptide sample; and (10) calculating the miR-224 content in the serum sample. The methoddisclosed by the present invention has high accuracy and reliable results.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Method for simultaneously detecting various residues of veterinary drug in meat food
InactiveCN109298109ASimplify preprocessing stepsReduce preprocessing timeComponent separationPhospholipidVeterinary Drugs
The invention relates to the technical field of the detection of residues of veterinary drug, and provides a method for simultaneously detecting various residues of veterinary drug in meat food. The method comprises the following steps of treating a sample, establishing a standard curve and calculating the content of each residue of veterinary drug in the detected sample, and the detection methodis an HPLC-MS (Ultra Performance Liquid Chromatography-Mass Spectrometry) method. Therefore, a PLS-A (Probabilistic Latent Semantic Analysis) solid phase extraction small column is adopted, activationand balance steps are not required, more than 95% of matrix disturbance substances, including protein, salt, phospholipid and the like, can be removed after extraction liquid directly passes throughthe small column, most veterinary drugs can directly pass without being retained, and therefore, different categories of residues of veterinary drug can be completely extracted only through preliminary treatment for one time. A preliminary treatment step is simplified, a solid phase extraction column does not need to be activated, in addition, various categories of residues of veterinary drug canbe simultaneously detected, preliminary treatment time is greatly shortened, and detection cost is lowered. A result indicates that the method is accurate, quick, convenient and reliable in quantitative results and is very suitable for simultaneously processing multiple batches of samples.
Owner:昌邑市检验检测中心
Aminated mesoporous silica-glucose-manganese dioxide nanocomposite and preparation method and application thereof
InactiveCN106771254ASmall sizeEasy to operateMaterial nanotechnologyBiological testingManganeseMesoporous silica
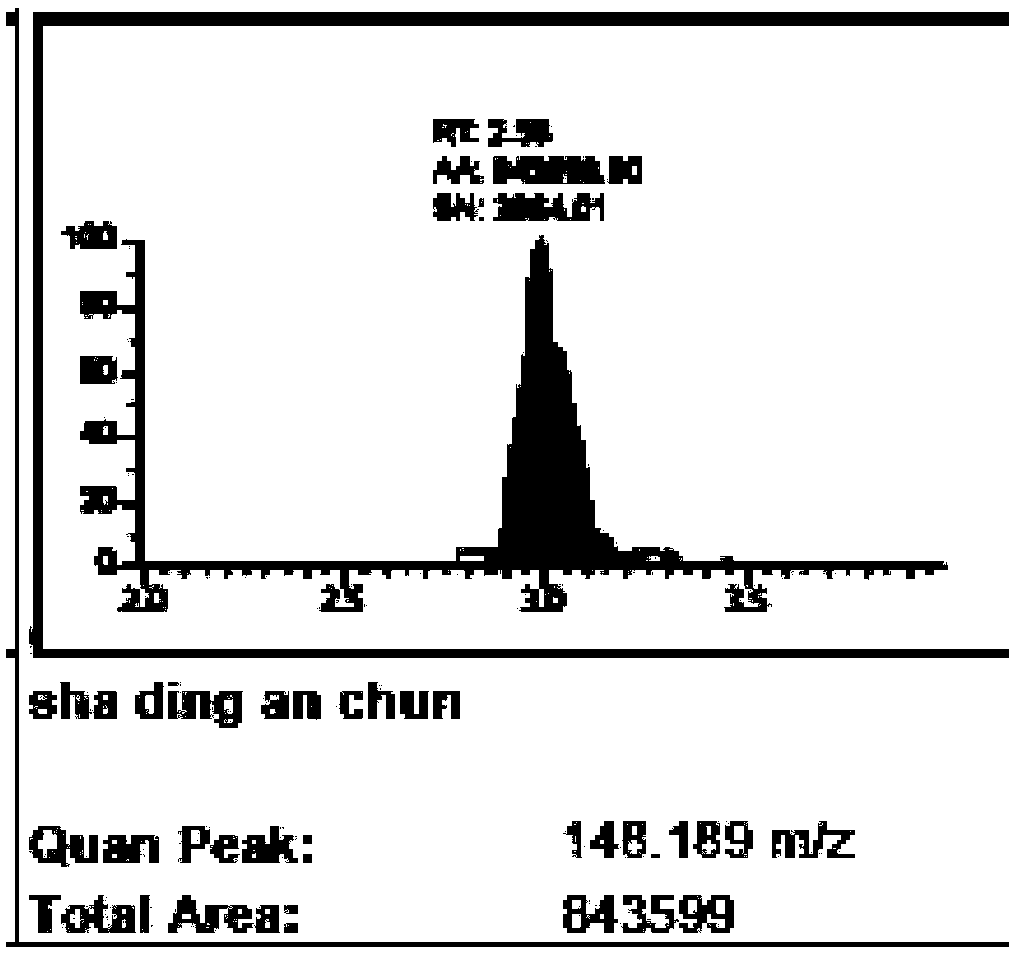
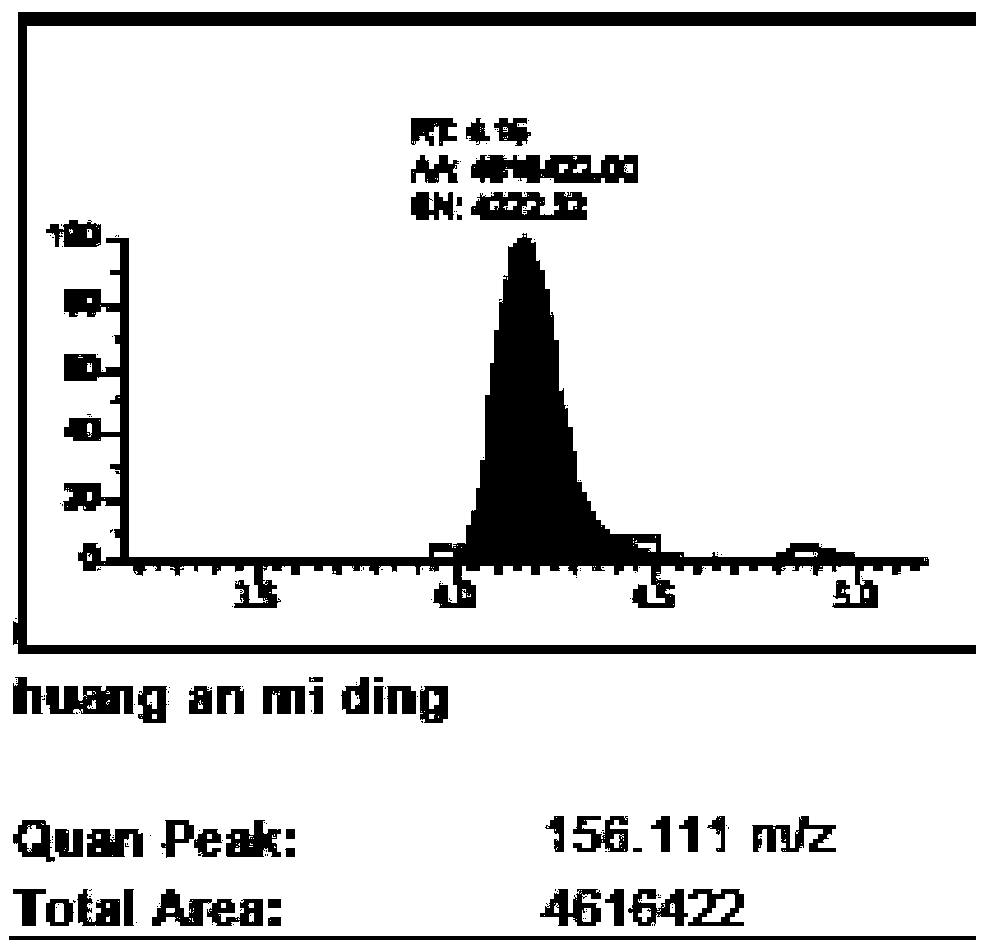
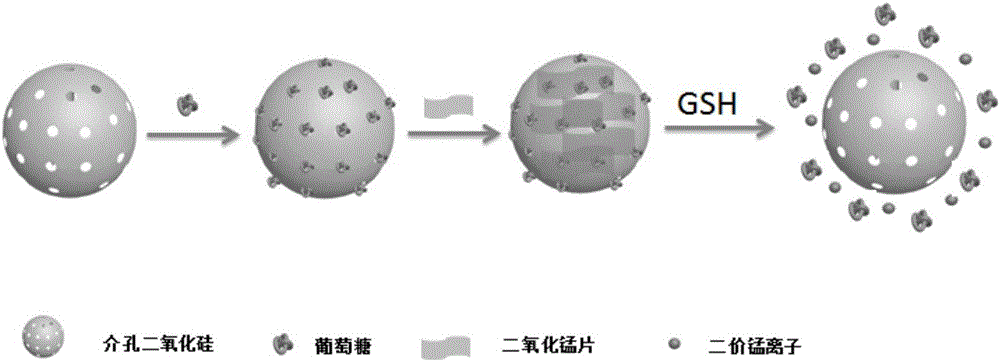
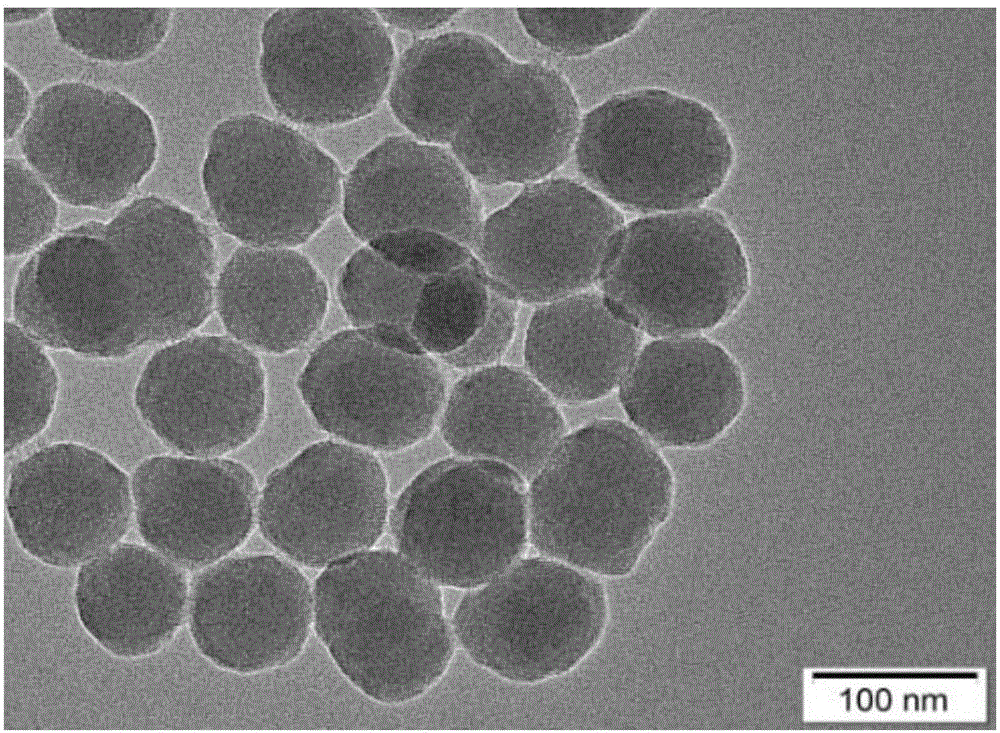
The invention belongs to the technical field of nano materials and particularly relates to aminated mesoporous silica-glucose-manganese dioxide nanocomposite and a preparation method and application thereof. The nanocomposite is prepared from manganese dioxide nanosheet and aminated mesoporous SiO2 nanosphere containing glucose, wherein the surface of the aminated mesoporous SiO2 nanosphere is coated by the manganese dioxide nanosheet, the particle size of the aminated mesoporous SiO2 nanosphere is 40 to 60nm, and the hole diameter is 2 to 3nm. The preparation method of the aminated mesoporous silica-glucose-manganese dioxide nanocomposite comprises the steps of preparing aminated mesoporous SiO2 and MnO2 nanosheet, then dispersing the aminated mesoporous SiO2 into a glucose water solution and finally connecting the MnO2 nanosheet to the surface of aminated MSN. According to the aminated mesoporous silica-glucose-manganese dioxide nanocomposite and the preparation method and application thereof, PGM is utilized to detect; thus, the content of GSH in a sample of the aminated mesoporous silica-glucose-manganese dioxide nanocomposite can be quantitatively detected, a detection line is low, and flexibility is high.
Owner:QUFU NORMAL UNIV
Method for in-situ measuring iron content in biological tissue sample by adopting isotopic dilution LA-ICP-MS (laser ablation inductively coupled plasma mass spectrometry)
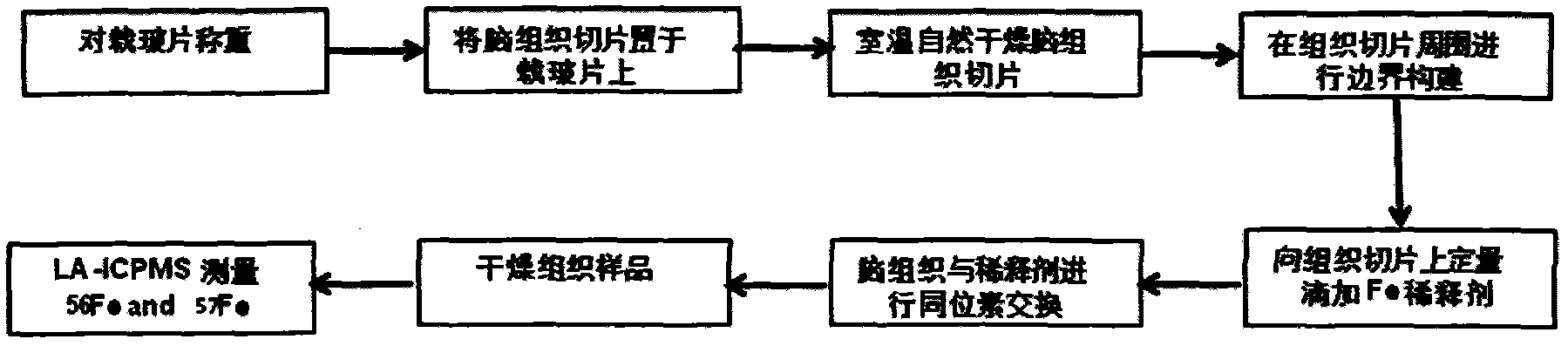
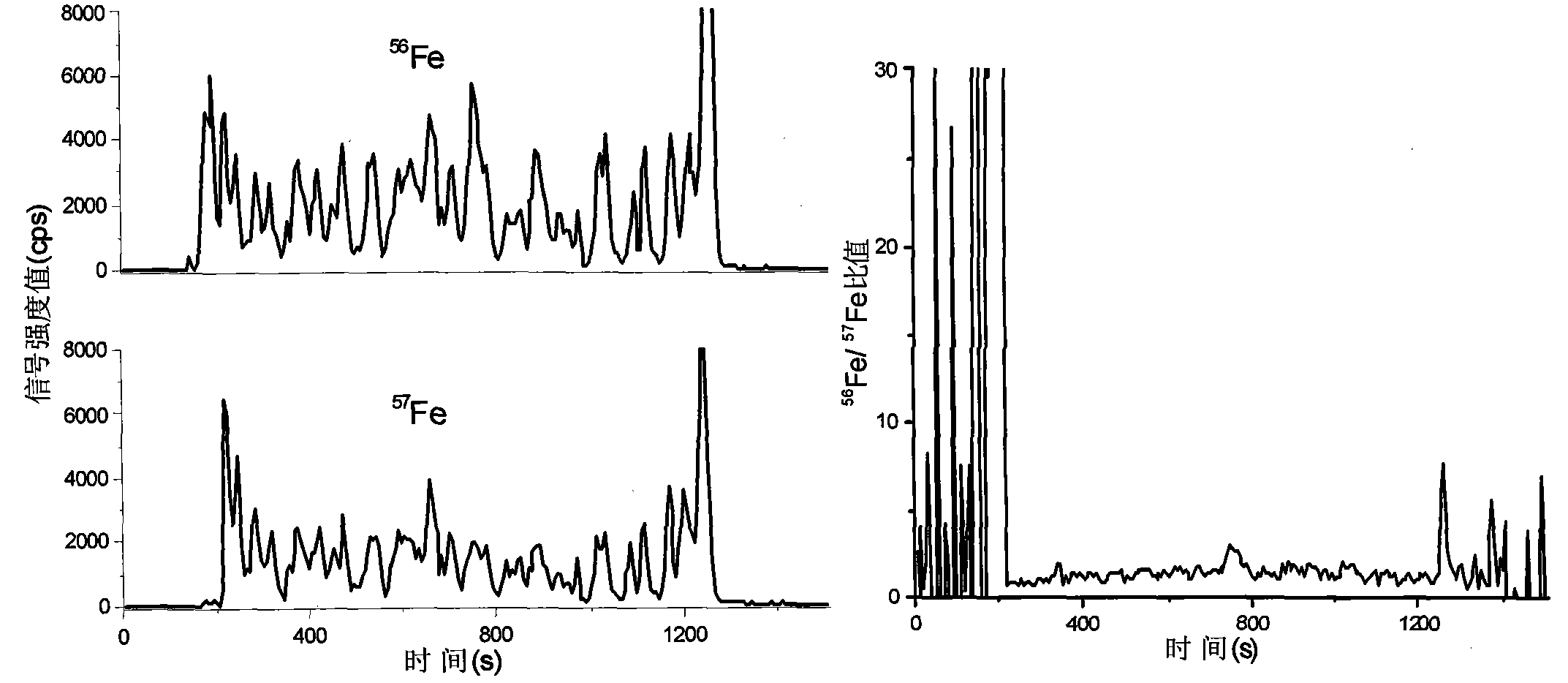
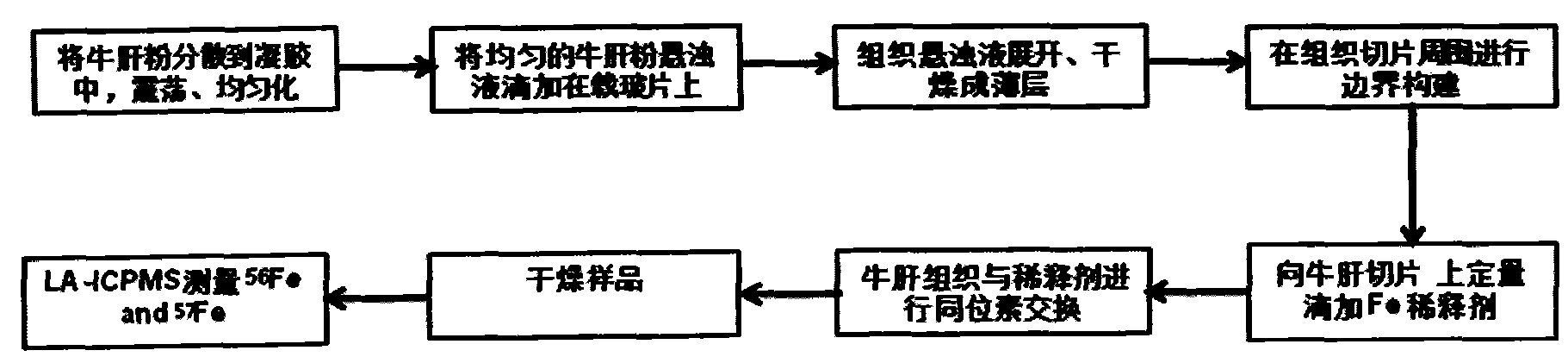
ActiveCN103837592AEasy to addOptimize equilibration timePreparing sample for investigationMaterial analysis by electric/magnetic meansLaser ablation inductively coupled plasma mass spectrometryMicrocell
The invention discloses a method for in-situ measuring iron content in a biological tissue sample by adopting isotopic dilution LA-ICP-MS (laser ablation inductively coupled plasma mass spectrometry). The method comprises the following steps of building a sample boundary by adopting a tissue anti-dropping material for a biological section sample, and adding quantitative isotopic dilution agent in a boundary to adequately exchange isotope of the tissue sample; optimizing the addition way of the isotopic dilution agent, the isotopic balance time and the condition of the in-situ isotopic ratio measurement technology; in-situ measuring the iron content in the uniform tissue section sample of goat brain and beef liver by adopting the isotopic dilution-LA-ICP-MS technology, wherein the measurement result is consistent with that of the microwave digestion-isotopic dilution method. The method can be further applied to the in-situ and microcell quantitative measurement and imaging analysis of metal elements in the biological tissue section sample in clinical process, and an important clinical significance for establishing the connection between the distribution variation of iron element in the tissue sample and the typical neurological disease can be realized.
Owner:NAT INST OF METROLOGY CHINA
Determination method of lycopene
InactiveCN104316610AGood linear relationshipMeet analysis requirementsComponent separationFood additiveHplc method
The invention relates to a determination method of lycopene, and belongs to the field of food additives and detection methods of the food additives. The determination method of the lycopene comprises the following steps: dissolving 1mg of standard substances by using a mobile phase, making volume up to 50mL, preparing a 20microg / mL standard solution, feeding 20microL of a sample under certain chromatographic conditions, regressing the concentration X by a corresponding peak area Y, and calculating to obtain a regression equation; and accurately weighing 0.2g of the sample, firstly adding a small amount of dichloromethane for dissolving, then making volume up to 200mL by using the mobile phase, feeding 20microL of the sample under the certain chromatographic conditions, and calculating the concentration according to the obtained corresponding peak area. The determination method determines the content of the lycopene by adopting an HPLC method, has the advantages of simplicity, convenience, accuracy, reliability and the like, and is suitable for being used in common laboratories.
Owner:西安莹朴生物科技股份有限公司
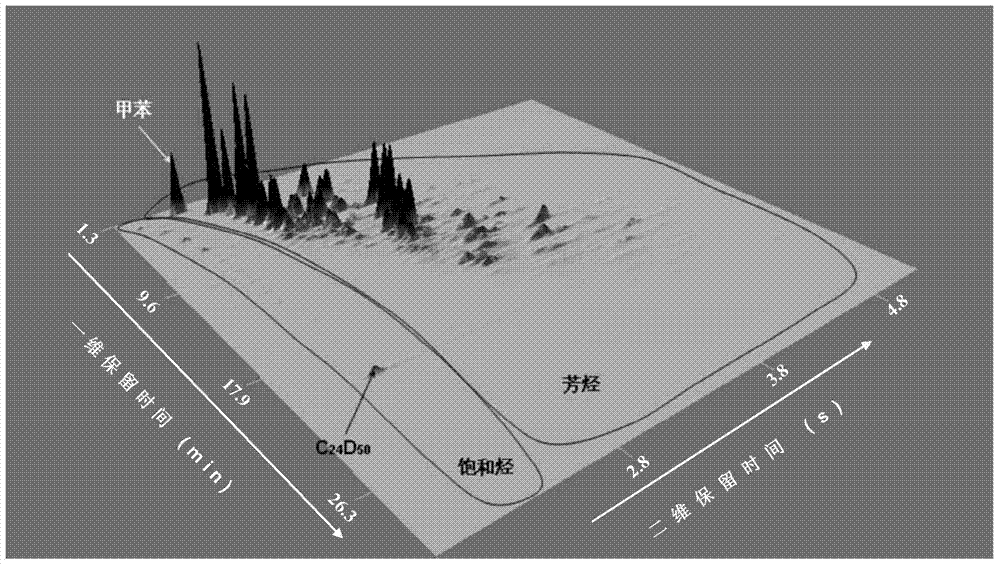
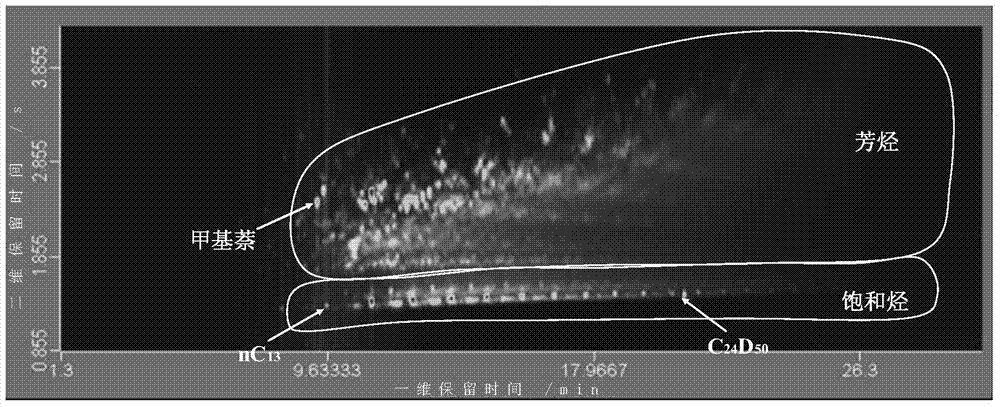
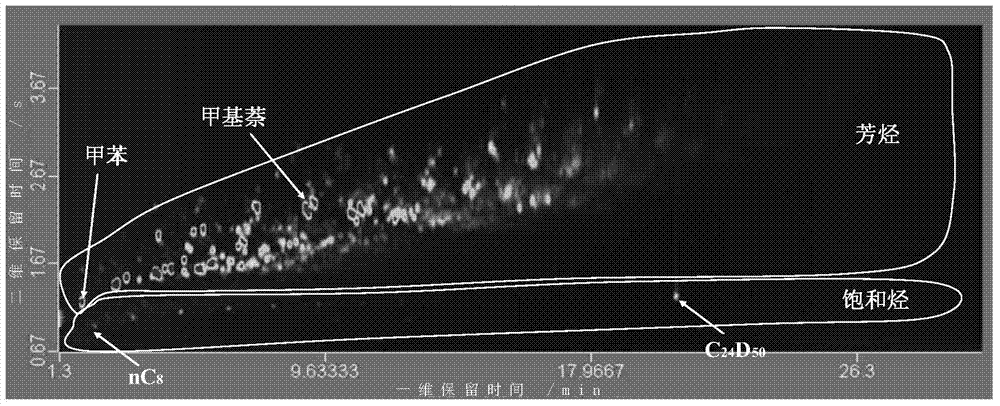
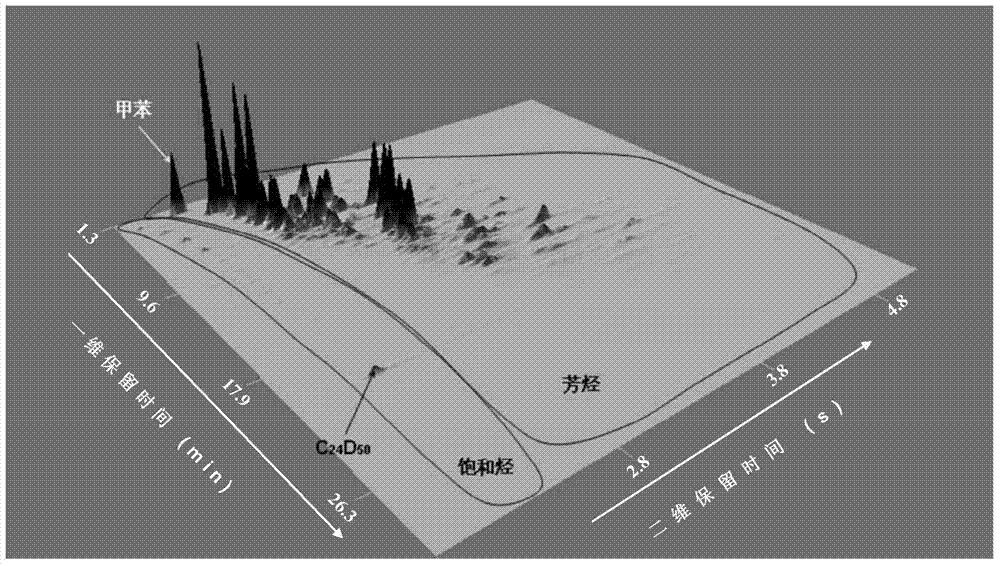
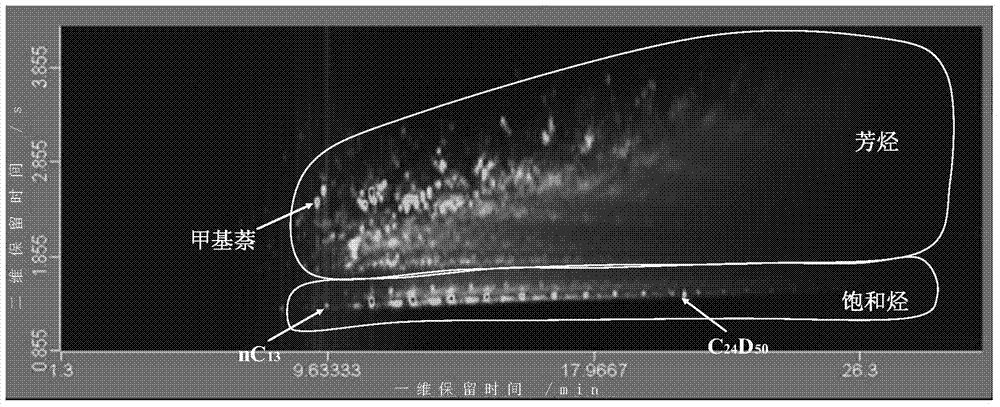
Quantitative analysis method of liquid-state products of hydrocarbon generation and expulsion simulation experiment
ActiveCN103592380AEliminate distractionsEliminate Double MeteringComponent separationHydrogenGas phase
The invention provides a quantitative analysis method of liquid-state products of a hydrocarbon generation and expulsion simulation experiment. The method comprises the following steps: collecting and treating the liquid-state products of the hydrocarbon generation and expulsion simulation experiment, wherein the step comprises light hydrocarbon collecting, heavy hydrocarbon collecting, standard sample adding, dehydrating, filtering and concentrating; carrying out quantitative analysis on the liquid-state products of the hydrocarbon generation and expulsion simulation experiment by utilizing a comprehensive two-dimensional gas chromatography-hydrogen flame ionization detector, wherein the step comprises primary comprehensive two-dimensional analysis, natural volatilization for constant weight as well as secondary comprehensive two-dimensional analysis; calculating full-component quantitative result of the liquid-state products of the hydrocarbon generation and expulsion simulation experiment. The quantitative analysis method disclosed by the invention can be used for avoiding volatilization of light hydrocarbon, has better experiment result repeatability and simple and easy-to-learn operation, and provides a reliable technical method for the quantitative analysis of the liquid-state products of the hydrocarbon generation and expulsion simulation experiment, so that estimation on basin oil and gas resource amount is more objective.
Owner:PETROCHINA CO LTD
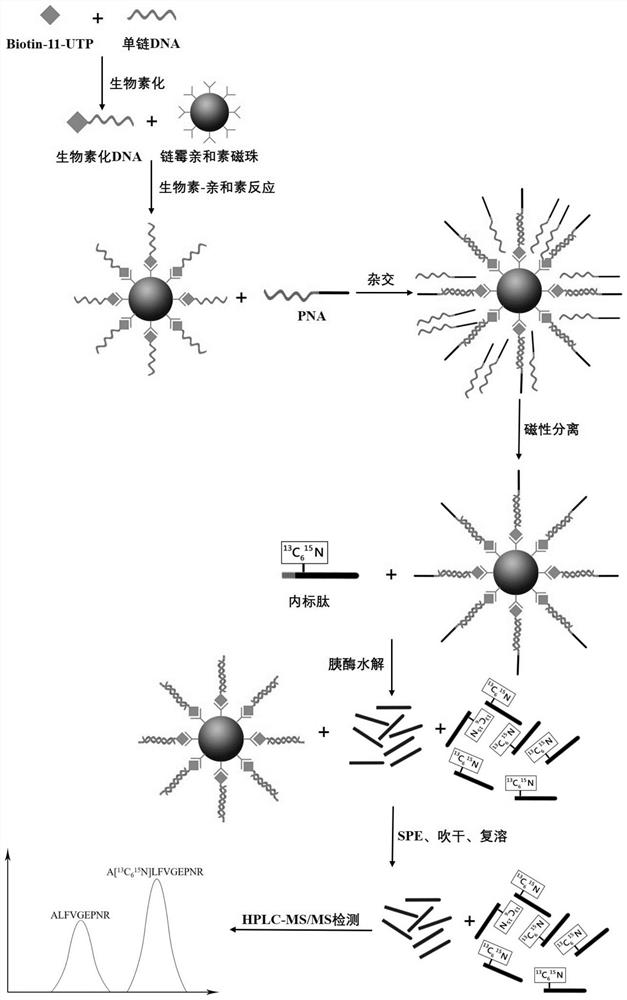
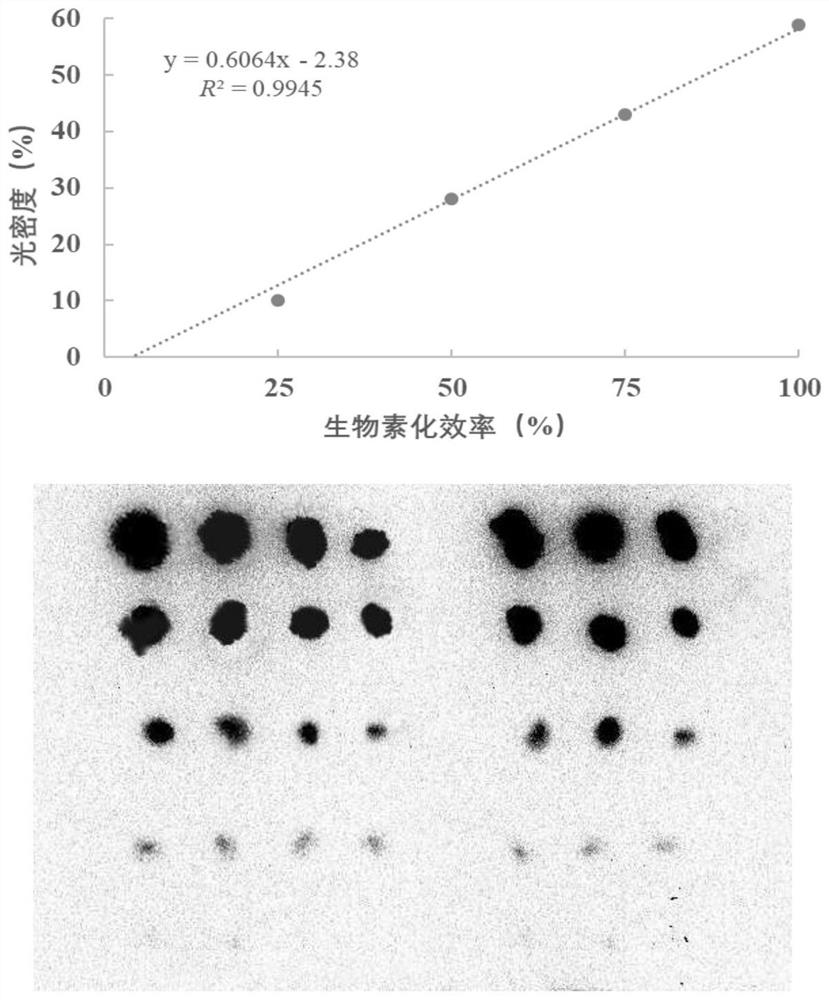
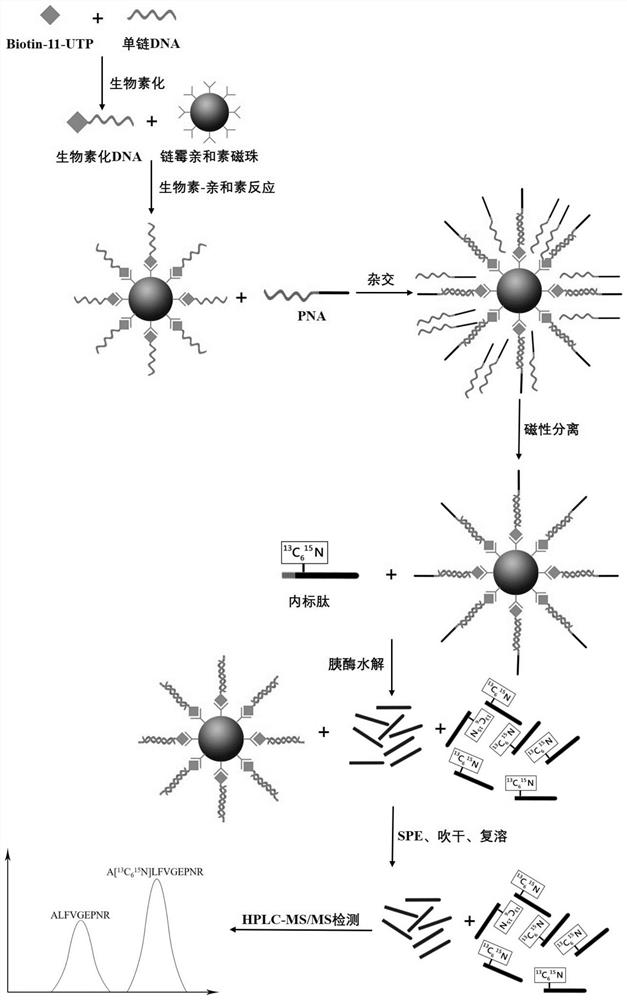
Method for quantitatively detecting content of free DNA by isotope dilution mass spectrometry
InactiveCN112342271AQuantitative results are accurate and reliableAvoid the effect of amplification efficiencyComponent separationMicrobiological testing/measurementMagnetic beadNucleic Acid Probes
The invention belongs to the technical field of biology, and particularly relates to a method for quantitatively detecting the content of free DNA through isotope dilution mass spectrometry. Firstly,a peptide nucleic acid probe is disclosed, the sequence of a nucleic acid part in the peptide nucleic acid probe is shown as SEQ ID NO: 1, and the sequence of a peptide part in the peptide nucleic acid probe is shown as SEQ ID NO: 2. The invention also discloses a method for quantitatively detecting the content of the free DNA single strand by isotope dilution mass spectrometry. The method comprises the following steps: forming a free DNA-biotin-streptavidin magnetic bead compound; adding a peptide nucleic acid probe to capture the free DNA-biotin-streptavidin magnetic bead compound; performing enzymolysis; performing re-dissolving; indirectly calculating the DNA content in the sample according to the peak area ratio; correcting the DNA concentration to obtain a final concentration value.The method for quantitatively detecting the content of the free DNA single strand by the isotope dilution mass spectrometry has the advantages of high accuracy, reliable result and the like.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
U6, miR 92a and miR 21 ternary RT-qPCR detecting method and kit
ActiveCN107012241ASimplified reverse transcription stepsAchieving single-tube triplex reverse transcriptionMicrobiological testing/measurementEnzyme systemPolymerase L
The invention belongs to the field of biological detection, and particularly relates to a detecting method for carrying out single-tube ternary reverse transcription on three kinds of miRNA including U6, miR 92a and miR 21, a detecting method for carrying out single-tube double quantification respectively after reverse transcription and a kit. The kit comprises a reverse transcription stem-loop primer group, a quantitative detecting primer group, a quantitative detecting probe group, an enzyme system and a PCR reaction reagent; reverse transcription of U6, miR 92a and miR 21 can be carried out in the same reaction tube; meanwhile, by high-sensitivity and high-specificity quantitative detecting primer sequences and quantitative detecting probes and PCR reaction procedures and conditions which are matched with the high-sensitivity and high-specificity quantitative detecting primer sequences and quantitative detecting probes, miR 21 and U6 as well as miR 92a and U6 can be subjected to single-tube double quantification, operation errors during single-tube single quantification in the prior art are avoided, and quantification is simple and accurate. In addition, a double-polymerase amplification for Stoffel fragments and Tfl DNA polymerase is introduced, while detection specificity is improved, lots of templates can be tolerated, and detection sensitivity and result stability are improved.
Owner:东莞微量精准检测研究院有限公司
A real-time fluorescent quantitative PCR kit and application for quantitative detection of krecs gene
ActiveCN103173535BEasy accessEasy to storeMicrobiological testing/measurementPcr ctppFluorescent pcr
The invention relates to a real-time fluorescence quantitative PCR (polymerase chain reaction) kit for quantitatively detecting KRECs gene and application thereof. The kit comprises a PCR system based on nested PCR technology and a real-time fluorescence quantitative PCR system based on real-time fluorescence PCR technology, wherein the nested PCR system comprises forward and reverse primers for KRECs and beta-actin genes; and the real-time fluorescence quantitative PCR system comprises forward and reverse primers and specific fluorescence probes for KRECs and beta-actin genes. The kit can be used for quickly screening the B-cell level of a neonatal immune system, and has the advantages of high sensitivity, high stability and excellent reproducibility. The method is suitable for quantitative detection of KRECs, can be used for screening functions of the neonatal immune system, and has practical clinical application value.
Owner:SHANGHAI ADVANCED CLINICAL LAB SCI
Facial nerve paralysis rehabilitation detection system based on artificial intelligence
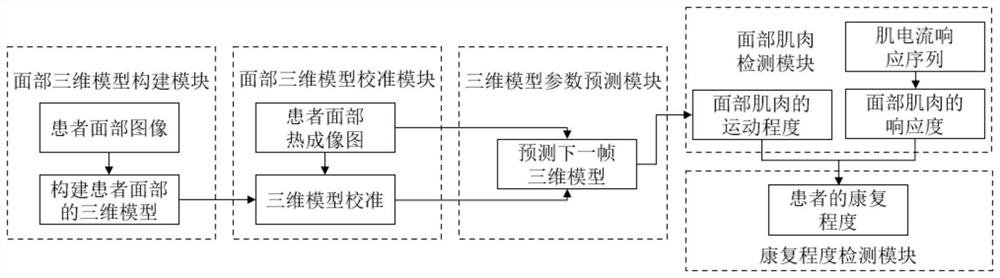
InactiveCN112562850AQuantitative results are accurate and reliableReduce power consumptionMedical automated diagnosisThree-dimensional object recognitionComputer visionThree dimensional model
The invention provides a facial nerve paralysis rehabilitation detection system based on artificial intelligence. The system comprises: a facial three-dimensional model construction module for constructing a three-dimensional model of the face of a patient according to an acquired facial image of the patient; a face three-dimensional model calibration module which is used for calibrating a three-dimensional model of the face of the patient based on the acquired face thermal imaging image of the patient; a three-dimensional model parameter prediction module which is used for predicting a next frame of three-dimensional model based on the patient face thermal imaging image and the parameters of the three-dimensional model; a facial muscle detection module which is used for obtaining the responsivity of facial muscles based on the obtained muscle current response sequence and obtaining the movement degree of the facial muscles based on the predicted three-dimensional model; and a rehabilitation degree detection module which is used for obtaining the rehabilitation degree of the patient based on the movement degree of the facial muscles and the responsivity of the facial muscles. The system can accurately quantify the rehabilitation degree of a patient and assist the patient in rehabilitation training.
Owner:黄振海
Real-time fluorescence quantitative PCR (polymerase chain reaction) kit for quantitatively detecting TRECs gene and application thereof
The invention relates to a real-time fluorescence quantitative PCR (polymerase chain reaction) kit for quantitatively detecting TRECs gene and application thereof. The kit comprises a PCR system based on nested PCR technology and a real-time fluorescence quantitative PCR system based on real-time fluorescence PCR technology, wherein the nested PCR system comprises forward and reverse primers for TRECs and beta-actin genes; and the real-time fluorescence quantitative PCR system comprises forward and reverse primers and specific fluorescence probes for TRECs and beta-actin genes. The kit can be used for quickly screening the T-cell level of a neonatal immune system, and has the advantages of high sensitivity, high stability and excellent reproducibility. The method is suitable for quantitative detection of TRECs, can be used for screening functions of the neonatal immune system, and has practical clinical application value.
Owner:无锡联合利康临床检验所有限公司
Rapid detection method for imidacloprid water body pollution by fluorescent quantitative PCR
ActiveCN109735606AHigh sensitivityAccurate measurementMicrobiological testing/measurementFluorescenceTotal rna
The invention relates to a water body pollutant detection method, in particular to a rapid detection method for imidacloprid water body pollution by fluorescence quantitative PCR, and belongs to the field of molecular biology. By detecting a response reaction of the aquatic insect choroterpes yixinggensis mitochondrial gene nad4L expression level to imidacloprid, imidacloprid pollution monitoringis conducted, and the method particularly comprises the following steps of 1, culture of the choroterpes yixinggensis of a to-be-detected water sample; 2, total RNA extraction; 3, gel electrophoresisdetection; 4, RNA concentration determination; 5, RNA inversion into cDNA; 6, standard construction and standard curve making; 7, gene expression quantity determination; 8, data analysis. The method is high in flexibility, good in timeliness, high in specificity, easy to implement, and capable of rapidly detecting whether or not the water body is polluted by the imidacloprid.
Owner:ZHEJIANG NORMAL UNIVERSITY
A triple RT-qPCR detection method and kit for u6, mir 92a and miR 21
ActiveCN107012241BSimplified reverse transcription stepsAchieving single-tube triplex reverse transcriptionMicrobiological testing/measurementEnzyme systemBiochemistry
The invention belongs to the field of biological detection, and particularly relates to a detecting method for carrying out single-tube ternary reverse transcription on three kinds of miRNA including U6, miR 92a and miR 21, a detecting method for carrying out single-tube double quantification respectively after reverse transcription and a kit. The kit comprises a reverse transcription stem-loop primer group, a quantitative detecting primer group, a quantitative detecting probe group, an enzyme system and a PCR reaction reagent; reverse transcription of U6, miR 92a and miR 21 can be carried out in the same reaction tube; meanwhile, by high-sensitivity and high-specificity quantitative detecting primer sequences and quantitative detecting probes and PCR reaction procedures and conditions which are matched with the high-sensitivity and high-specificity quantitative detecting primer sequences and quantitative detecting probes, miR 21 and U6 as well as miR 92a and U6 can be subjected to single-tube double quantification, operation errors during single-tube single quantification in the prior art are avoided, and quantification is simple and accurate. In addition, a double-polymerase amplification for Stoffel fragments and Tfl DNA polymerase is introduced, while detection specificity is improved, lots of templates can be tolerated, and detection sensitivity and result stability are improved.
Owner:东莞微量精准检测研究院有限公司
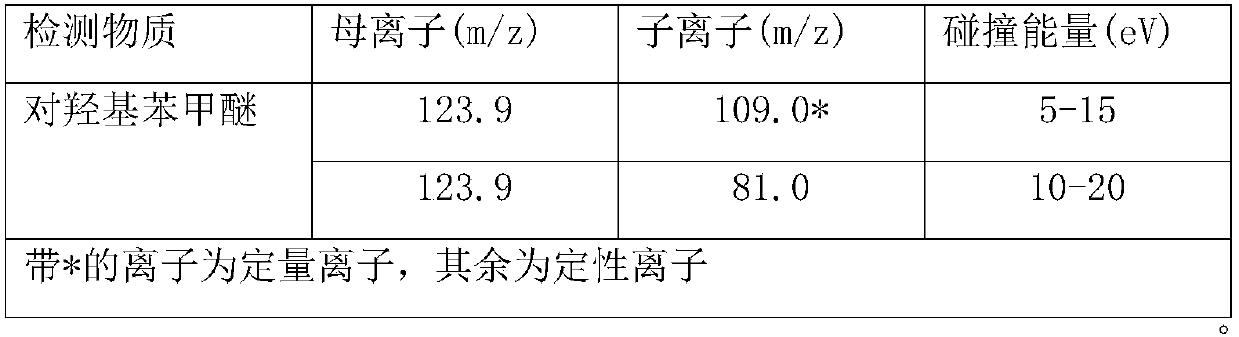
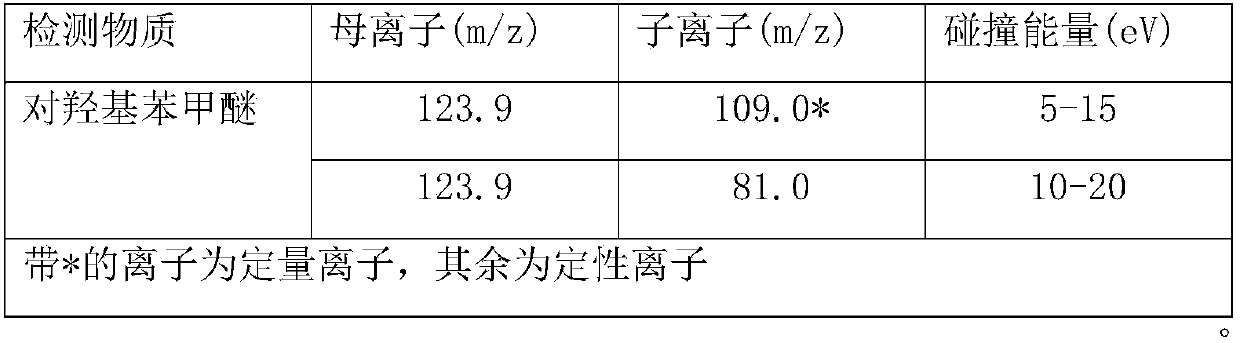
Method for measuring transporting quantity of para-hydroxy benzaldehyde in food contact material
InactiveCN105954436AQuantitative results are accurate and reliableHigh precisionComponent separationLipid formationWater based
The invention relates to a method for measuring transporting quantity of para-hydroxy benzaldehyde in a food contact material, and belongs to the technical field of analysis and detection. The method for measuring transporting quantity of para-hydroxy benzaldehyde in the food contact material is set up, quantification results are accurate and reliable, and accuracy, stability and the automation degree are high. According to stipulations of national standards in China, feed simulants are selected to simulate different foods for transporting tests, and the feed simulants comprise water, an acetic acid solution with the mass concentration of 3%, an ethanol solution with the volume fraction of 10% and isooctane which represent water-based, acidic, alcoholic and lipid foods respectively; the method is wide in coverage and high in practicability.
Owner:CHANGZHOU INST OF TECH
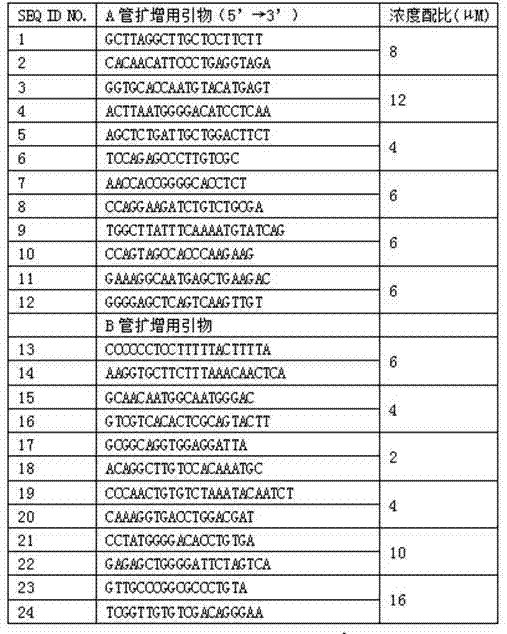
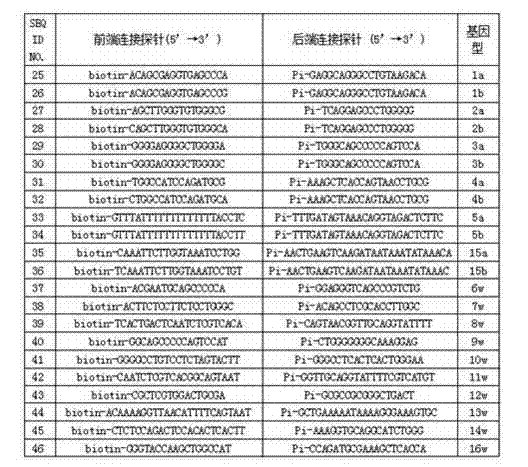
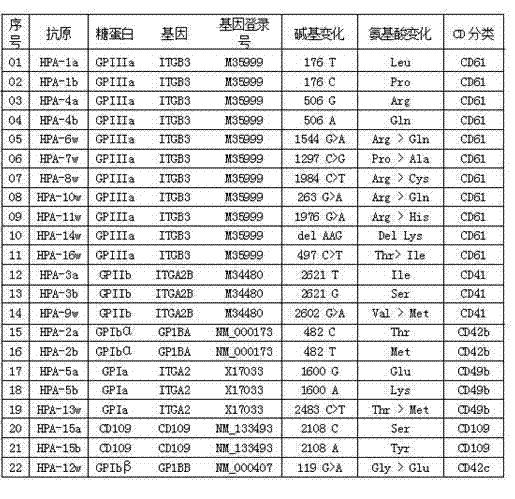
Human platelet antigen genotyping liquid chip and human platelet antigen genotyping detection method thereof
InactiveCN102230017BImprove throughputIncreased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceHybridization reactionGene
The invention belongs to the technical field of molecular biology, and in particular relates to a human platelet antigen genotyping liquid phase chip and a detection method thereof. Human platelet antigen genotyping technology is an important technical means to provide patients with matching platelets and promote the safety and effectiveness of clinical platelet transfusion. However, the existing human platelet antigen genotyping technology has low throughput and high accuracy. Indexes such as sensitivity and sensitivity need to be improved. The liquid phase chip of the present invention includes primers 1-24, connection probes 25-46 and fluorescently encoded microspheres coated with detection probes 47-68, and the human platelet antigen gene is completed through amplification, connection and hybridization reactions. Typing detection. The liquid phase chip and detection method adopted in the present invention have the advantages of large flux, high sensitivity, strong specificity, good repeatability, wide linear range, simple operation, strong flexibility, and wide application range, and are clinically accurate, Efficient and practical human platelet antigen genotyping detection method.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Primer probes, kits and methods for precise quantitative detection of specific gene components of transgenic rice Kefeng No. 6 strain
ActiveCN107312819BReliable resultsQuantitative results are accurate and reliableMicrobiological testing/measurementDNA/RNA fragmentationGene ComponentNucleotide
The invention relates to an oligonucleotide primer probe for precise quantitative detection of specific gene components of the transgenic rice Kefeng No. 6 strain, and a kit comprising the primer probe. The present invention also relates to a digital PCR detection method for quantitatively detecting the specific gene components of the transgenic rice Kefeng No. 6 strain, said method comprising using specific oligonucleotide primers and Fluorescently labeled probes. The invention also relates to the application of the specific oligonucleotide primers and fluorescent probes for the specific genes of the transgenic rice strains in the quantitative detection of the specific gene components of the transgenic rice Kefeng No. 6 strain. The digital PCR detection method of the present invention can accurately and sensitively measure the content of specific gene components of the transgenic rice Kefeng No. 6 strain in the sample, and the sensitivity can reach 1 copy / μL.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
A Quantitative Analysis Method Integrating Proteome and Glycoproteome
ActiveCN110702916BThe steps are simple and time-savingEasy to operateBiological testingUltrafiltrationBinding peptide
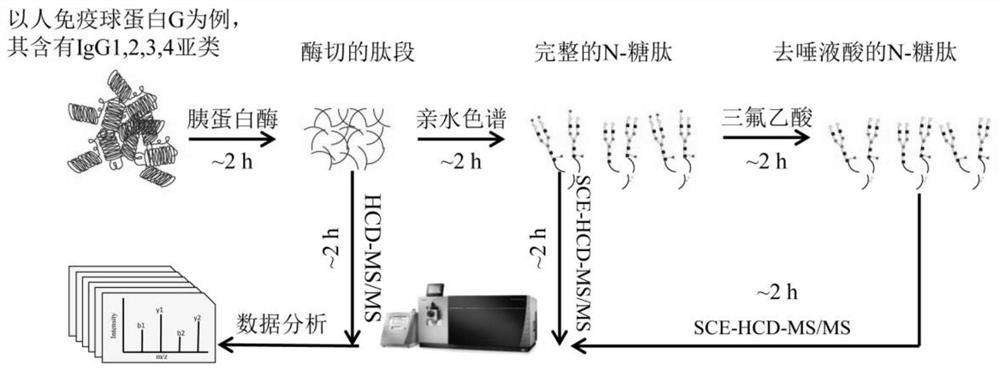
The invention provides a quantitative analysis method for integrating proteome and glycoproteome, which comprises the following steps: (1) performing reductive alkylation treatment after heating and denaturing the protein; (2) using trypsin in an ultrafiltration tube to convert the protein Cut peptides with enzymes and centrifuge to obtain peptides; (3) Hydrophilic interaction chromatography HILIC filler binds peptides, washes and removes impurities, and then elutes to obtain complete N-glycopeptides; (4) Adds trifluoroacetic acid and heats to remove N-glycopeptides ‑Sialic acid on the periphery of the sugar chain to obtain asialic N‑glycopeptide; (5) Use HCD‑MS / MS to analyze the peptide to obtain proteome data, and use MaxQuant for qualitative and quantitative analysis; (6) Use SCE‑HCD‑MS / MS analysis of intact N-glycopeptides and asialo-N-glycopeptides, using Xcalibur software for qualitative and quantitative analysis. The invention can simply, quickly and effectively realize the quantitative analysis of proteome and glycoproteome, and has a good application prospect in the study of the occurrence and development mechanism of diseases and the discovery of new biomarkers.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
A real-time fluorescent quantitative PCR kit for quantitatively detecting trecs and krecs genes and its application
ActiveCN103173533BEasy accessEasy to storeMicrobiological testing/measurementFluorescence/phosphorescenceFluorescent pcrPcr ctpp
The invention relates to a real-time fluorescence quantitative PCR (polymerase chain reaction) kit for quantitatively detecting TRECs and KRECs genes and application thereof. The kit comprises a PCR system based on nested PCR technology and a real-time fluorescence quantitative PCR system based on real-time fluorescence PCR technology, wherein the nested PCR system comprises forward and reverse primers for TRECs, KRECs and beta-actin genes; and the real-time fluorescence quantitative PCR system comprises forward and reverse primers and specific fluorescence probes for TRECs, KRECs and beta-actin genes. The kit can be used for quickly screening the T-cell level and B-cell level of a neonatal immune system, and has the advantages of high sensitivity, high stability and excellent reproducibility. The method is suitable for combined quantitative detection of TRECs and KRECs, can be used for screening functions of the neonatal immune system, and has practical clinical application value.
Owner:无锡联合利康临床检验所有限公司
Primer probe, kit and method for accurate and quantitative detection of internal standard of transgenic rice
InactiveCN107312818AAvoid false positivesReliable resultsMicrobiological testing/measurementDNA/RNA fragmentationOligonucleotide primersInternal standard
The present invention relates to an oligonucleotide primer probe for accurate and quantitative detection of a transgenic rice internal standard sucrose phosphate synthase (SPS) gene component, and a kit containing the primer probe. The present invention further relates to a digital PCR detection method for quantitatively detecting the transgenic rice internal standard gene SPS component, wherein the method comprises using the specific oligonucleotide primer and the fluorescent label probe for the transgenic rice internal standard gene SPS. The invention further relates to applications of the specific oligonucleotide primer and the fluorescent probe for the specific gene of the transgenic rice line in quantitative detection of the transgenic rice internal standard gene SPS component. According to the present invention, by using the digital PCR detection method, the transgenic rice internal standard gene SPS component content in the sample can be accurately and sensitively determined, and the absolute detection sensitivity can achieve 1 copy / [mu]L.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Fluorescent quantitative PCR method for rapid detection of imidacloprid water pollution
ActiveCN109735606BHigh sensitivityAccurate measurementMicrobiological testing/measurementTotal rnaImidacloprid
The invention relates to a water body pollutant detection method, in particular to a rapid detection method for imidacloprid water body pollution by fluorescent quantitative PCR, belonging to the field of molecular biology. The present invention detects the mitochondrial gene of the aquatic insect Yixing Kuanjima wxya The technology of imidacloprid pollution monitoring based on the response of expression level to imidacloprid, the specific implementation steps are as follows: (1) cultivation of Yixing broad-based mayfia in water samples to be tested; (2) extraction of total RNA; (3) gel electrophoresis detection ; (4) Measurement of RNA concentration; (5) Inversion of RNA into cDNA; (6) Construction of standard products and creation of standard curve; (7) Measurement of gene expression; (8) Data analysis. The method has high sensitivity, good timeliness, strong specificity, simple operation, and can quickly detect whether the water body is polluted by imidacloprid.
Owner:ZHEJIANG NORMAL UNIVERSITY
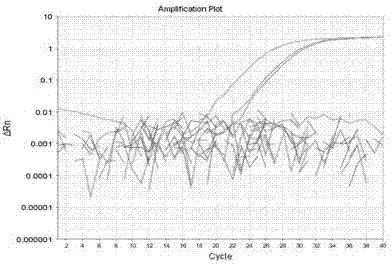
A genome editing detection method, kit and application
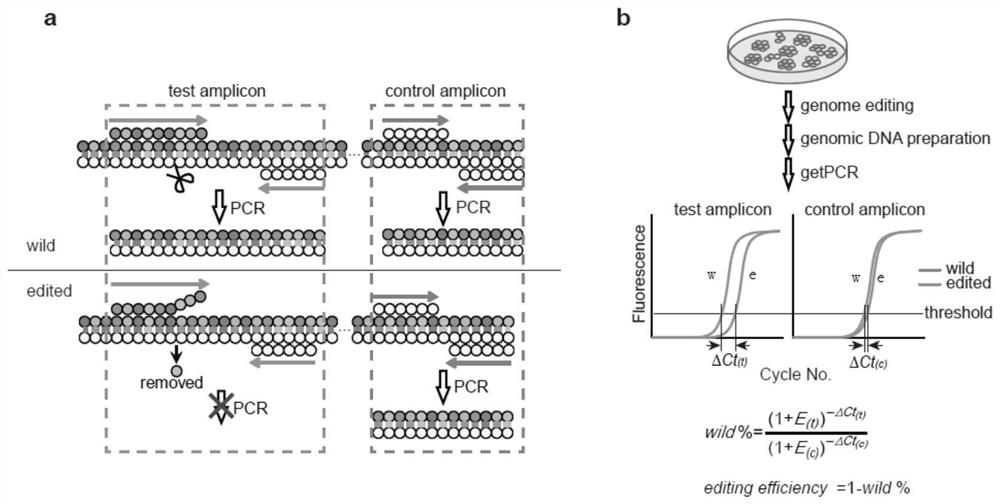
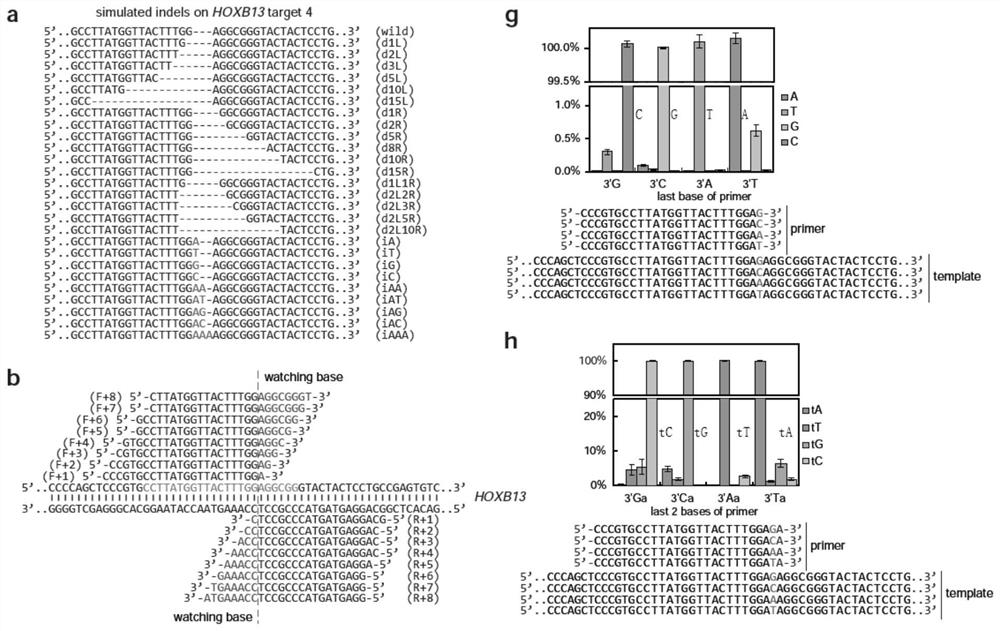
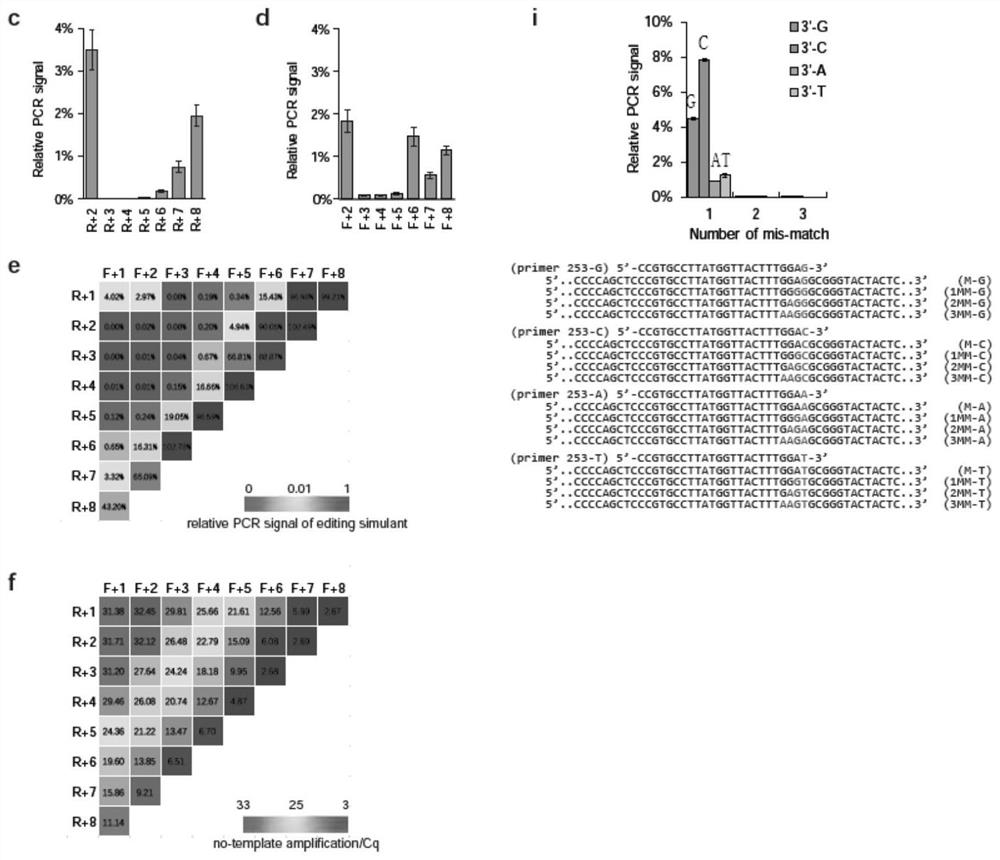
ActiveCN110607356BSimple Editing Efficiency Evaluation MethodAccurate and Reliable Evaluation Method of Editing EfficiencyMicrobiological testing/measurementBase JGenome editing
The disclosure belongs to the field of genome editing efficiency detection, and specifically relates to a genome editing detection method, kit and application. In view of the defects of gene editing efficiency detection methods in the prior art, such as Sanger, NGS, and methods based on mismatch-specific nucleases, etc. have defects such as complicated operation, high cost, and insufficient detection accuracy, the present disclosure provides a new method , called getPCR, based on Taq DNA polymerase specificity and real-time PCR technology, quantifies the wild-type DNA in the genome to be tested, and confirms the genome editing efficiency by calculating the percentage of wild-type DNA. This disclosed research provides the design rules of the corresponding guarded bases and optimized getPCR operation parameters. It has been verified that the method has good detection accuracy and is easy to operate. It can be applied to all genome editing methods to quantify genome editing efficiency, and can also be applied to Screening of single cell clones.
Owner:SHANDONG UNIV +1
Method for quantitatively detecting content of free DNA (deoxyribonucleic acid) by isotope dilution mass spectrometry
PendingCN113684254AQuantitative results are accurate and reliableAvoid the effect of amplification efficiencyComponent separationMicrobiological testing/measurementMagnetic beadNucleic Acid Probes
The invention belongs to the technical field of biology, and particularly relates to a method for quantitatively detecting the content of free DNA by isotope dilution mass spectrometry. Firstly, a peptide nucleic acid probe is disclosed, wherein the sequence of a nucleic acid part in the peptide nucleic acid probe is shown as SEQ ID NO: 1, and the sequence of a peptide part in the peptide nucleic acid probe is shown as SEQ ID NO: 2. The invention also discloses the method for quantitatively detecting the content of the free DNA single chain by the isotope dilution mass spectrometry. The method comprises the following steps of forming a free DNA-biotin-streptavidin magnetic bead compound; adding the peptide nucleic acid probe to capture the free DNA-biotin-streptavidin magnetic bead compound; performing enzymolysis; performing redissolving; indirectly calculating the DNA content in the sample according to the peak area ratio; and correcting the DNA concentration to obtain a final concentration value, and the like. The method for quantitatively detecting the content of the free DNA single chain by the isotope dilution mass spectrometry has the advantages of high accuracy, reliable result and the like.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
A paper-based enzyme-linked immunosorbent assay based on covalent bonding to immobilize capture antibodies
ActiveCN112098652BEasy maintenanceEasy to operateDisease diagnosisBiological testingClinical examReceptor
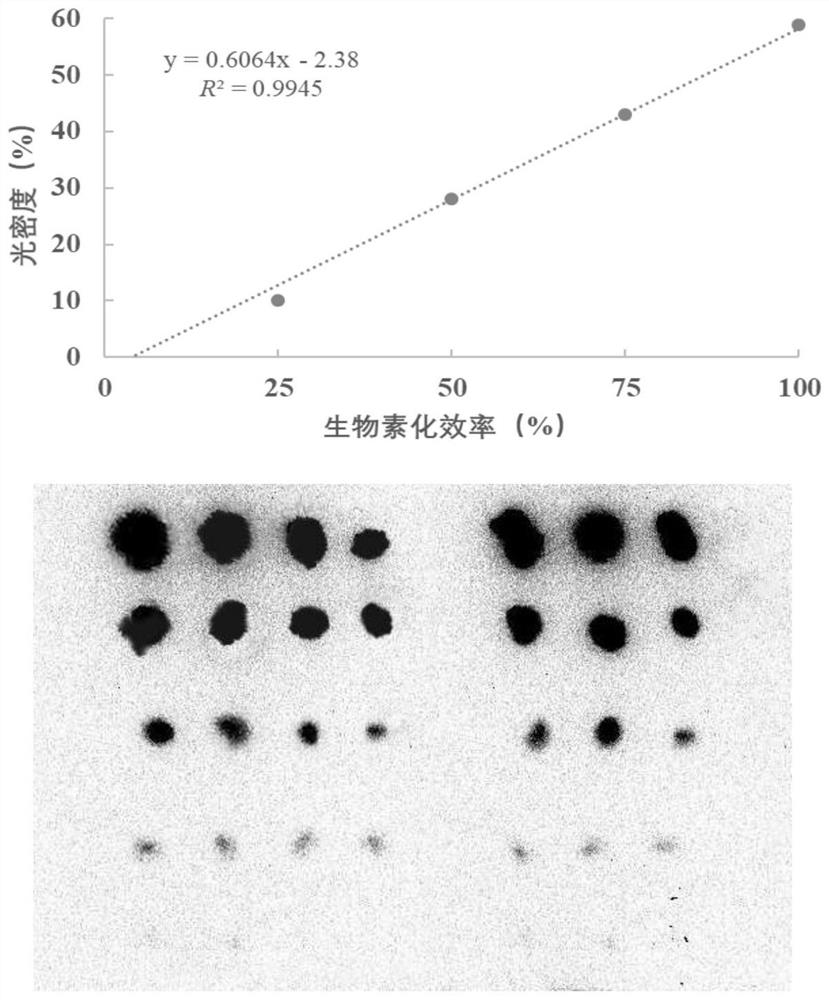
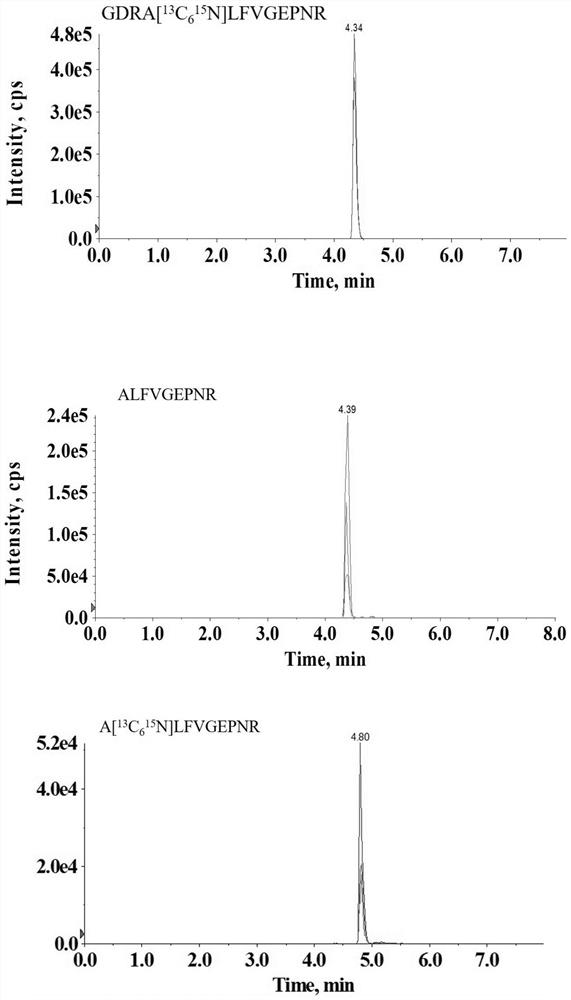
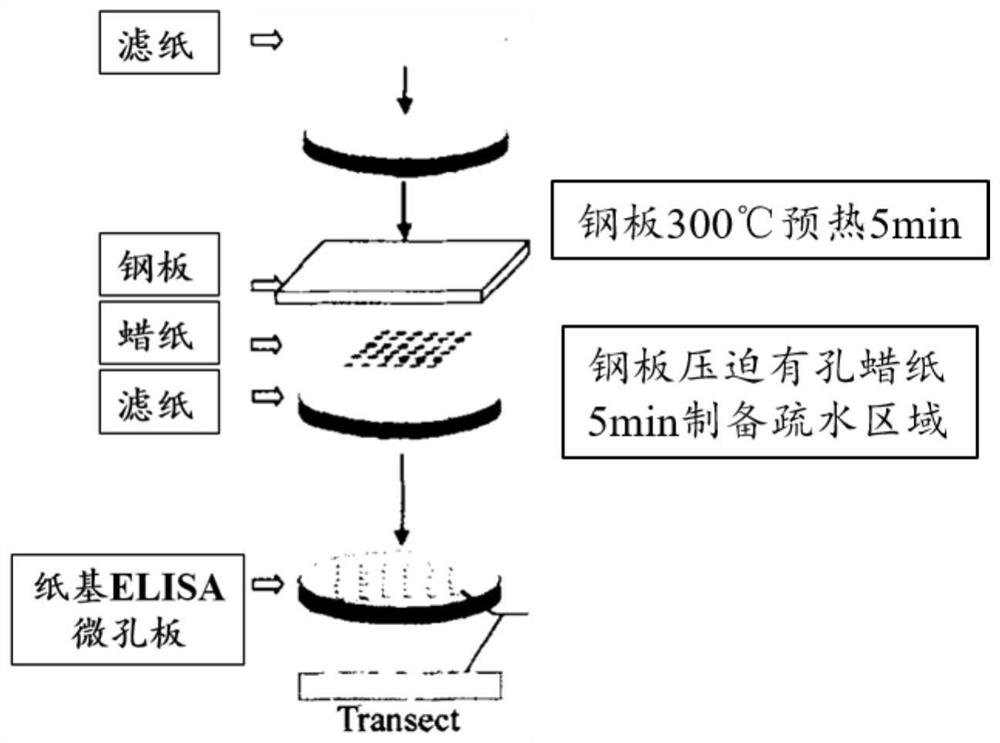
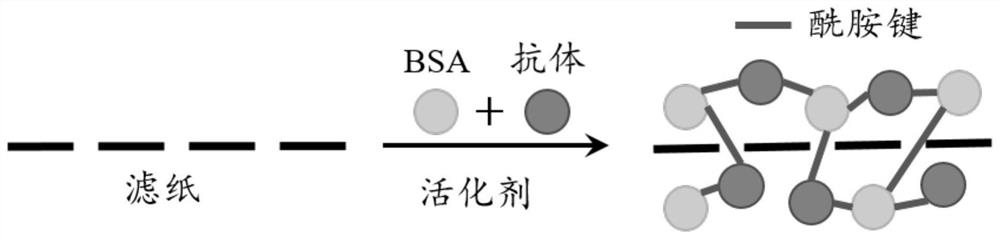
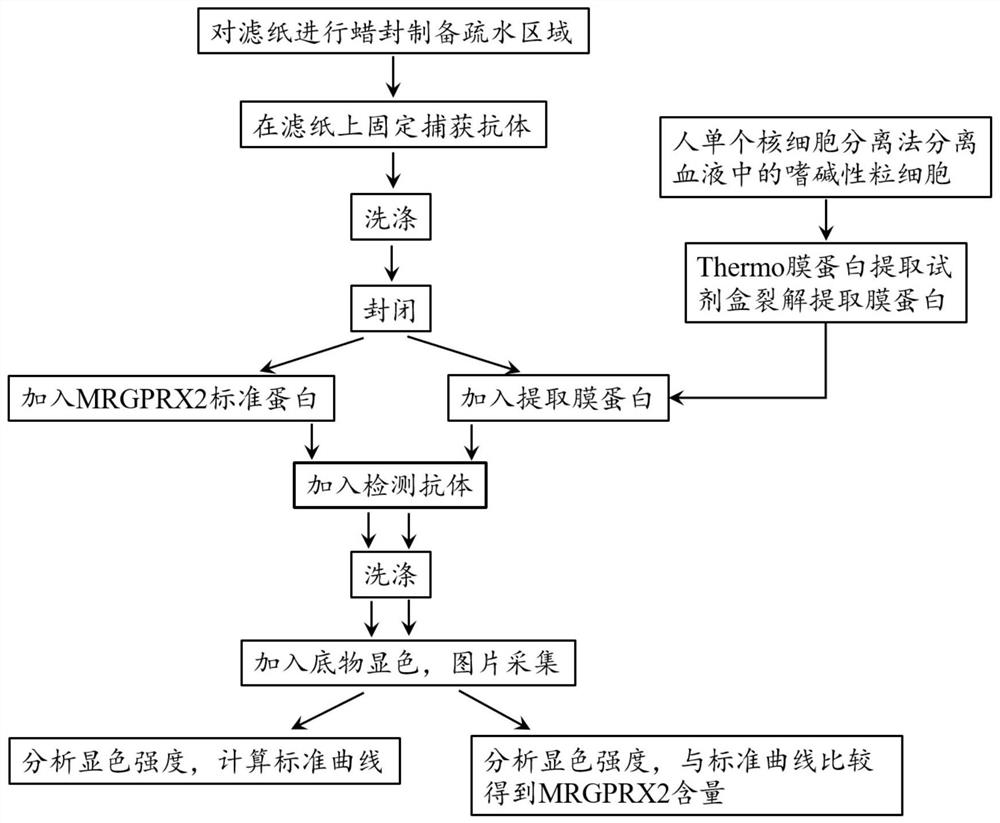
The invention discloses a paper-based double-antibody sandwich method based on a covalent bonding method to fix and capture antibodies, belongs to the technical field of immune analysis, and is used for detecting the content of allergy-like related MRGPRX2 receptors in human blood. Using MRGPRX2 mouse monoclonal antibody as the capture antibody, without the need to chemically modify the surface of the filter paper, by introducing bovine serum albumin (BSA) molecules with both carboxyl and amino functional groups, using the amide condensation reaction between the antibody molecule and the amino and carboxyl groups in the BSA molecule The antibody-BSA network structure was formed to realize the immobilization of the capture antibody; the MRGPRX2 rabbit polyclonal antibody labeled with horseradish peroxidase HRP was used as the detection antibody, and the MRGPRX2 solution was used as the standard to establish a paper-based double-antibody sandwich method for MRGPRX2. The immobilization method of the capture antibody is simple, efficient, and low in cost. The technical indicators of the detection method are high in detection throughput, fast in speed, detection limit and precision, etc., and the accuracy and reproducibility of the quantitative results are good. It is suitable for For clinical examination and blood epidemiological investigation.
Owner:XI AN JIAOTONG UNIV
Quantitative analysis method of liquid-state products of hydrocarbon generation and expulsion simulation experiment
ActiveCN103592380BEliminate distractionsEliminate Double MeteringComponent separationHydrogenGas phase
The invention provides a quantitative analysis method of liquid-state products of a hydrocarbon generation and expulsion simulation experiment. The method comprises the following steps: collecting and treating the liquid-state products of the hydrocarbon generation and expulsion simulation experiment, wherein the step comprises light hydrocarbon collecting, heavy hydrocarbon collecting, standard sample adding, dehydrating, filtering and concentrating; carrying out quantitative analysis on the liquid-state products of the hydrocarbon generation and expulsion simulation experiment by utilizing a comprehensive two-dimensional gas chromatography-hydrogen flame ionization detector, wherein the step comprises primary comprehensive two-dimensional analysis, natural volatilization for constant weight as well as secondary comprehensive two-dimensional analysis; calculating full-component quantitative result of the liquid-state products of the hydrocarbon generation and expulsion simulation experiment. The quantitative analysis method disclosed by the invention can be used for avoiding volatilization of light hydrocarbon, has better experiment result repeatability and simple and easy-to-learn operation, and provides a reliable technical method for the quantitative analysis of the liquid-state products of the hydrocarbon generation and expulsion simulation experiment, so that estimation on basin oil and gas resource amount is more objective.
Owner:PETROCHINA CO LTD
A fluorescent microsphere for labeling specific high-affinity recombinant antibody and its application
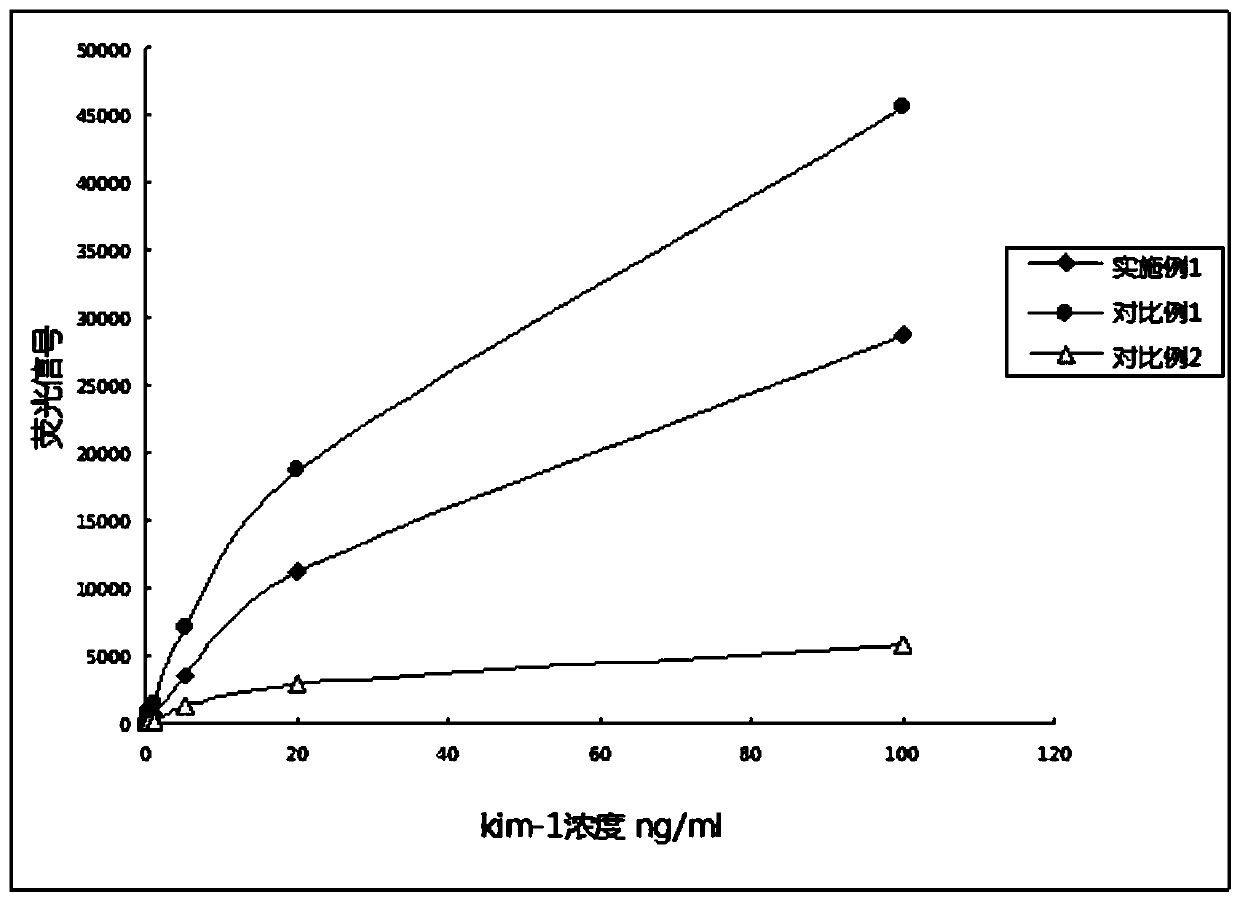
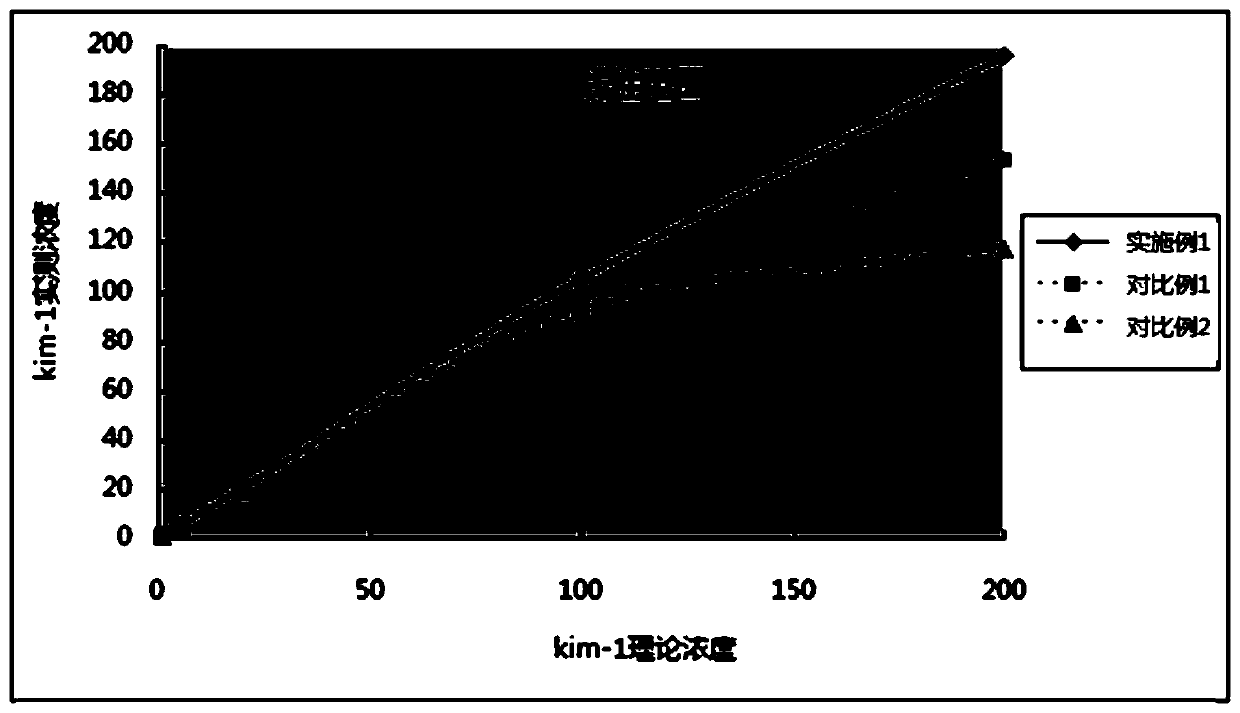
ActiveCN107271692BAppreciable fluorescence signal intensityImprove response offlineBiological testingLower limitImmunofluorescence
The invention belongs to the field of biological medicine, relates to a specific high-affinity recombinant antibody marking fluorescent microsphere and a preparation method and application thereof and particularly relates to a plug-and-play fluorescence detection card for quick quantitative detection of KIM-1, NGAL, IGFBP7, TIMP-2, IL-18, PCT and cTnI in human urine or blood samples. The method includes: activating fluorescent microspheres; adding the activated fluorescent microspheres into a dialysis bag filled with phosphate buffer to obtain dialyzed fluorescent microsphere solution; adding amino-polyethylene glycol-carboxyl, further activating the fluorescent microsphere solution subjected to secondary dialysis, making with a recombinant antibody, centrifuging after reaction, washing, precipitating, resuspending and precipitating. The fluorescent microsphere has advantages that immunofluorescence reaction lower limit can be remarkably improved to enable sensitivity of a prepared test card to be improved by 10-100 times of that of test cards prepared according to a traditional method, and a test range is remarkably expanded.
Owner:江苏博华医药科技有限公司
Primer probe, kit and method for accurate and quantitative detection of specific gene component of transgenic rice oryza sativa l line
InactiveCN107312817AReliable resultsQuantitative results are accurate and reliableMicrobiological testing/measurementDNA/RNA fragmentationOligonucleotide primersFluorescence
The present invention relates to an oligonucleotide primer probe for accurate and quantitative detection of the specific gene component of a transgenic rice oryza sativa l line, and a kit containing the primer probe. The invention further relates to a digital PCR detection method for quantitatively detecting the specific gene component of a transgenic rice oryza sativa l line, wherein the method comprises using the specific oligonucleotide primer and the fluorescent label probe for the specific gene of the transgenic rice oryza sativa l line. The present invention further relates to applications of the specific oligonucleotide primer and the fluorescent probe for the specific gene of the transgenic rice line in quantitative detection of the specific gene component of the transgenic rice oryza sativa l line. According to the present invention, by using the digital PCR detection method, the content of the specific gene component of the transgenic rice oryza sativa l line in the sample can be accurately and sensitively determined, and the sensitivity can achieve 0.5 copies / [mu]L.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com