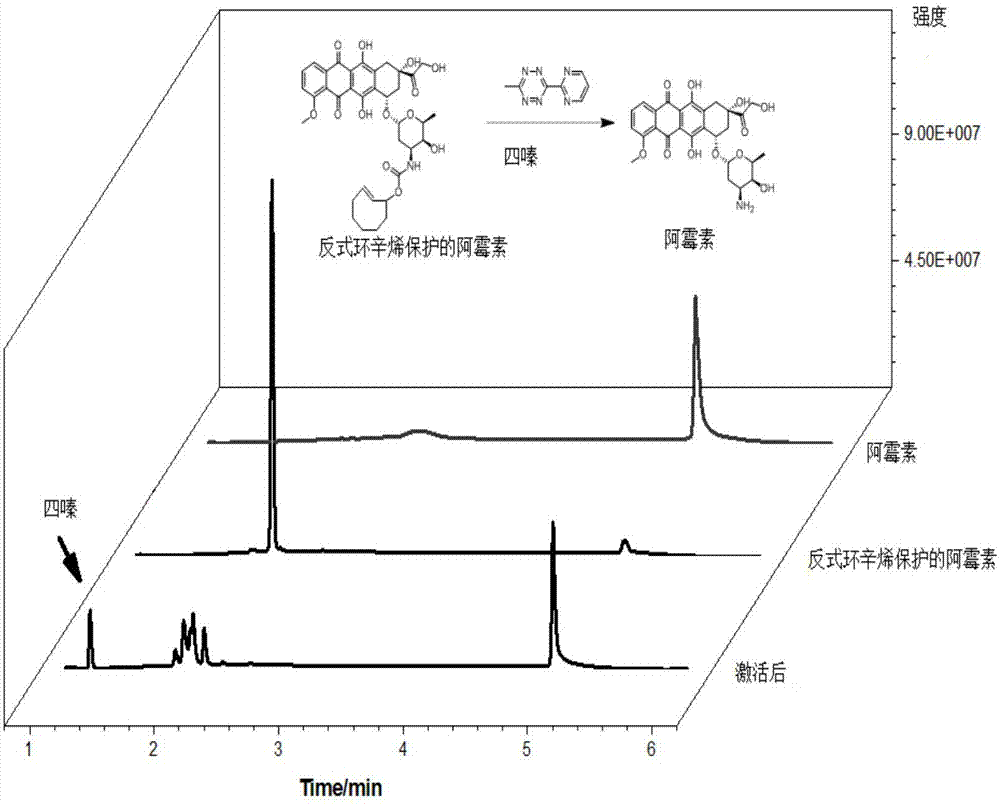
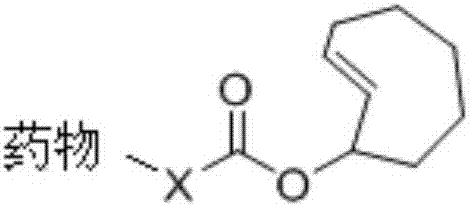
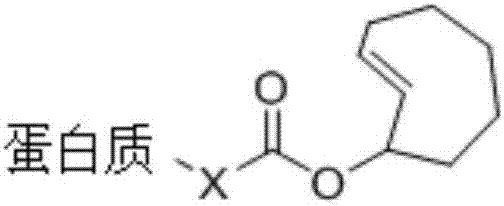
Application of asymmetrical 1,2,4,5-tetrazine molecule
An asymmetric and molecular technology, applied in 1 field, can solve the problems of limited application prospects, low efficiency, slow activation rate, etc., and achieve high activation efficiency and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation of 3-(2-pyrimidine)-6-methyl-1,2,4,5-tetrazine molecule
[0032] Mix 1.0mmol cyano-based substrate 2-cyanopyrimidine, 10.0mmol cyano-based substrate acetonitrile, 25mmol hydrazine hydrate, and 0.5mmol zinc trifluoromethanesulfonate in a round bottom flask and stir with a magnetic stirrer. The reaction conditions are vacuum nitrogen protection, The reaction was carried out at 60°C for 24 hours, then cooled to room temperature, slowly added dropwise a 1M sodium nitrite solution to the reaction system in an ice-water bath, adjusted the pH to 3 with a 1M aqueous hydrochloric acid solution, and then added 20 mL of dichloromethane Extracted three times, combined the organic phase, dried with anhydrous sodium sulfate, removed the organic solvent with a rotary evaporator, and finally separated and purified by column chromatography to obtain a red solid 3-(2-pyrimidine)-6-methyl-1, 2,4,5-tetrazine molecule, 40% yield. Characterization data: 1 H NMR (CDCl 3 ,400...
Embodiment 2
[0039] Example 2: In vitro activation of luciferase fLuc
[0040] fLuc is a firefly luciferase that converts luciferin to oxyluciferin and emits fluorescence at the same time. First, trans-cyclooctene-protected lysine (TCOK) was inserted into the 529 position of fLuc to obtain the protein fLuc-K529TCOK to inactivate fLuc, and then after the cells expressed the fLuc-K529TCOK protein, the cells were broken and purified to obtain pure fLuc- K529TCOK protein, followed by adding 50 μM of 3-(2-pyrimidine)-6-methyl-1,2,4,5-tetrazine molecules (the preparation method is the same as in Example 1) to the protein solution to make fLuc- K529TCOK is deprotected, and the activated fLuc catalyzes the conversion of luciferin to oxyluciferin, and produces fluorescence at the same time, and can activate fLuc-K529TCOK protein into wild-type protein fLuc within 4 minutes, and the activation efficiency can reach more than 90%.
Embodiment 3
[0041] Example 3: Activation of luciferase fLuc
[0042] First, insert trans-cyclooctene-protected lysine (TCOK) at position 529 of fLuc to obtain protein fLuc-K529TCOK can inactivate fLuc, and the activity of fLuc is completely inhibited. Add 50uM 3 to living HEK293T cells -(2-pyrimidine)-6-methyl-1,2,4,5-tetrazine molecule (the preparation method is the same as in Example 1) deprotects fLuc-K529TCOK, and the activated fLuc catalyzes the conversion of luciferin to oxidation Luciferin produces fluorescence at the same time, and can fully activate fLuc-K529TCOK within 4 minutes, and the activation efficiency can reach more than 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com