Serum replacement for immune cell suspending culture
A serum substitute and immune cell technology, applied in the direction of cell culture active agent, blood/immune system cells, culture process, etc., can solve the problems of strong pertinence, low cell yield, poor storage and application of medium, and achieve The ingredients are clear, the protein content is low, and the effect of avoiding repeated freezing and thawing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1. Preparation of Serum Substitute for Immune Cell Suspension Culture
[0030] Serum replacement formula A of the present invention is shown in Table 1.
[0031] Table 1: Formulation of the Serum Replacement of the Invention (Formulation A)
[0032]
[0033] The preparation method of the serum substitute is as follows:
[0034] 1. Prepare iron-saturated human transferrin solution:
[0035] (1) 40mg FeCl 3 Dissolved in 20ml of 1mM HCl solution, aliquoted and stored at -20°C to obtain No. 1 storage solution;
[0036] (2) Take 200 mg of human transferrin, dissolve it in 20 ml of RPMI1640 medium, add 300 μl of No. 1 stock solution and stir evenly to obtain an iron-saturated human transferrin solution (10 mg / ml), store at -30 °C save.
[0037] 2. Preparation of cholesterol solution: Weigh 58 mg of cholesterol powder, add it to 20 ml of RPMI1640 medium, shake it at 4°C for 1 hour with an ultrasonic oscillator to make it completely emulsified, and then obtain a...
Embodiment 2
[0042] Embodiment 2. Preparation of the serum-free medium comprising the serum substitute of the present invention
[0043] 1. Prepare commercially available medium according to the instructions to obtain RPMI 1640 basal medium (RPMI Medium 1640 medium, Beijing Oriental Huahui Biomedical Technology Co., Ltd.). The formulation of RPMI 1640 basal medium is shown in Table 2.
[0044] 2. The formulation of the serum-free medium containing the serum substitute of the present invention: the serum-free medium (medium A) of the present invention comprises 90% of RPMI 1640 basal medium and 10% of serum substitute A.
[0045] 3. Preparation of serum-free medium (medium A): before use, slowly add 100ml serum substitute A to 800ml RPMI 1640 basal medium, stir well, adjust the pH to 7.2 with 5% NaHCO3, and then add RPMI1640 The volume of the culture medium was supplemented to 1000 ml to obtain a serum-free medium (medium A) containing the serum substitute A of the present invention.
[0...
Embodiment 3
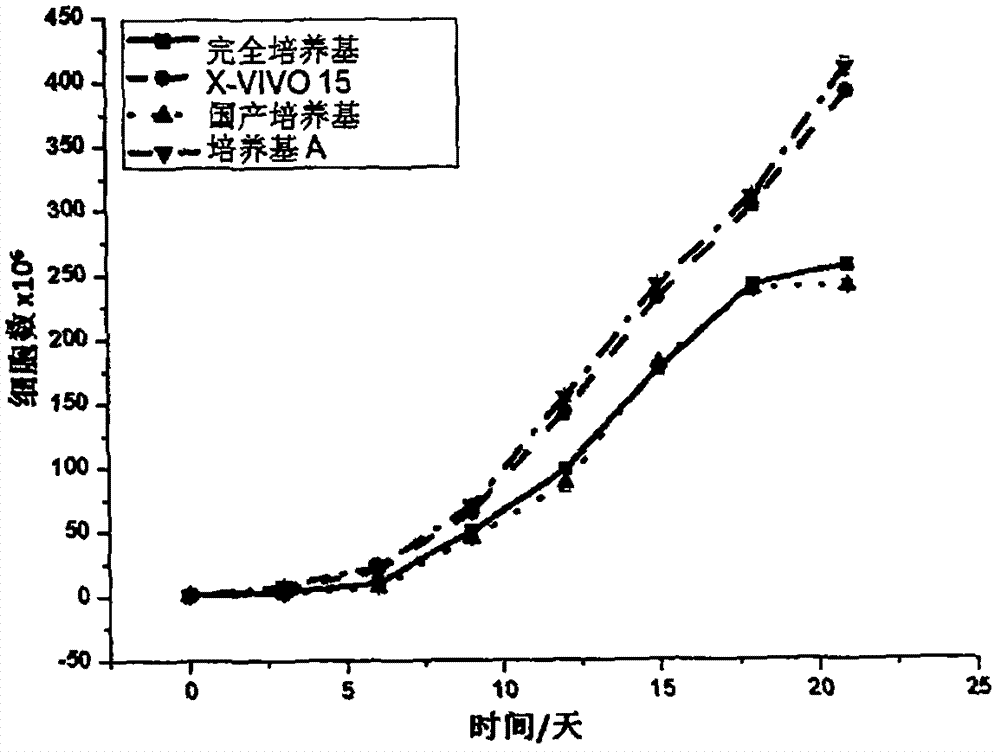
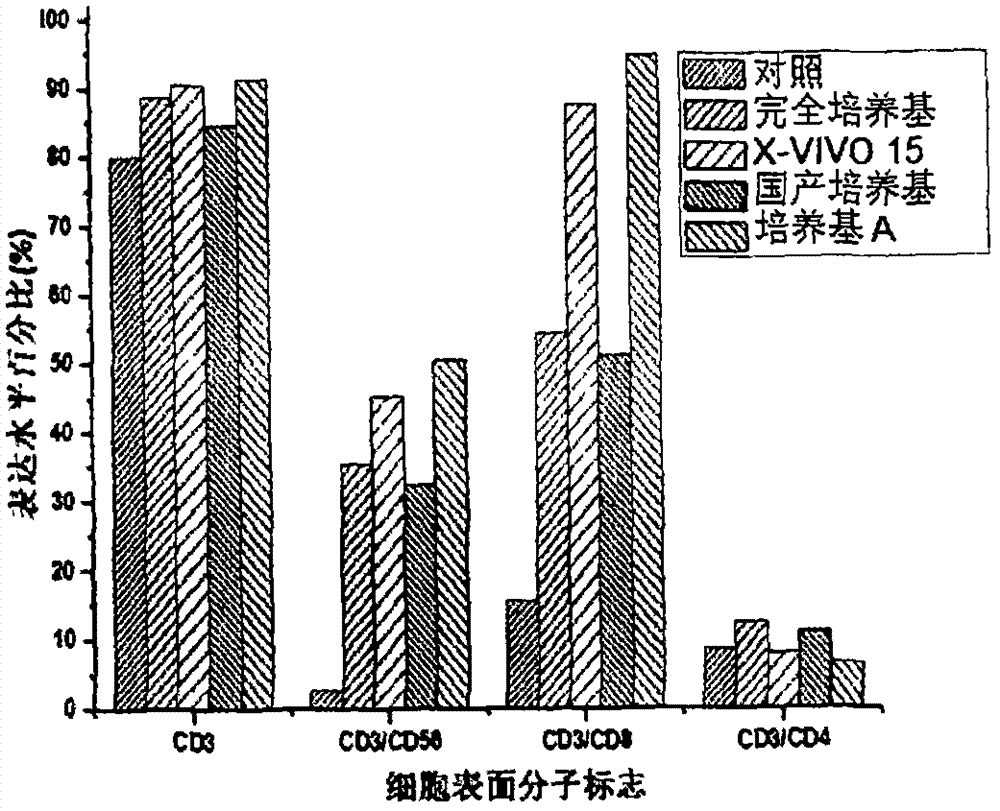
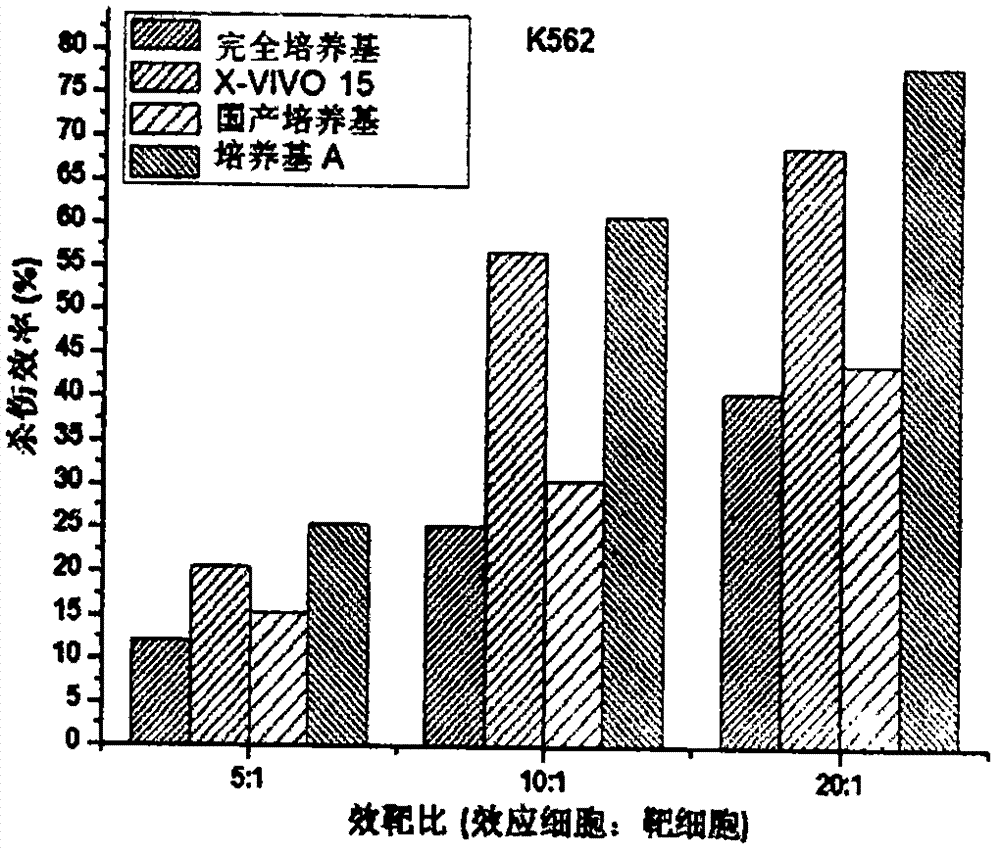
[0048] Example 3. Evaluation of the proliferation ability of CIK cells cultured in serum-free medium A
[0049] Peripheral blood mononuclear cells (PBMC) were isolated and purified according to the methods reported in the literature using peripheral blood from healthy people, and induced in vitro to prepare CIK cells. The experiment is divided into 4 groups (Table 3), using respectively: 1) serum-free medium medium A (medium A) of the present invention, 2) complete medium (RPMI 1640+10%FBS), 3) imported serum-free Medium (X-VIVO 15 (LONZA)) and 4) domestic serum-free medium for cell culture.
[0050] After PBMC purification, the cell number was adjusted to 5 × 10 6 / ml, inoculated into T175 culture flasks. From the first day of induction, the medium was changed in half every 3 days, and the final concentration of cytokines in the medium was kept constant. At the same time, samples were taken and counted by the trypan blue exclusion method until the end of the 21st day of cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com