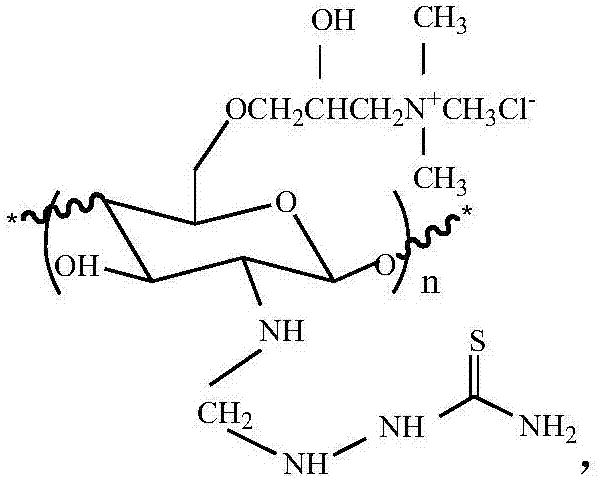
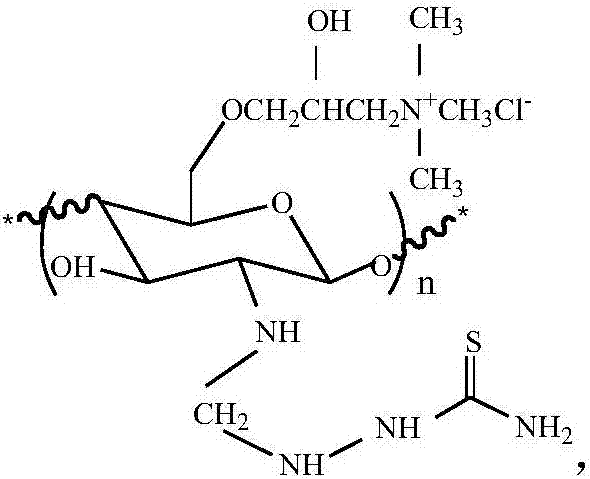
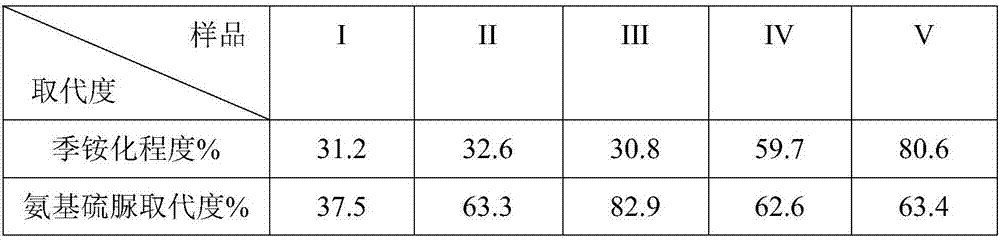
N-thiosemicarbazide-O-quaternary ammonium chitosan oligosaccharide and preparation method and application thereof
A technology of thiosemicarbazide and salt chitosan oligosaccharide is applied in the preparation of sugar derivatives, botanical equipment and methods, applications, etc., and can solve the problems of inability to use for a long time, easy to be dissolved and corroded, poor adsorption performance of chitosan oligosaccharide, etc. To achieve the effect of improving bacteriostatic performance, good metal adsorption and complexing ability and bacteriostatic ability, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] (1) Preparation of N-methylene chitosan oligosaccharides: Chitosan oligosaccharides are prepared into chitosan oligosaccharide aqueous solution, formaldehyde solution is added dropwise to the chitosan oligosaccharide aqueous solution, after the addition is completed, stir at room temperature for a period of time to obtain N- Methylene chitooligosaccharides;
[0030] (2) Preparation of N-methylene-O-quaternary ammonium chitosan oligosaccharide: the N-methylene chitosan oligosaccharide prepared in step (1) is added to an organic solvent for swelling, and then the aqueous solution of alkali is added to continue swelling, After heating, add 3-chloro-2-hydroxypropyltrimethylammonium chloride, and stir to get N-methylene-O-quaternary ammonium chitosan oligosaccharide;
[0031] (3) Synthesis of N-thiosemicarbazide-O-quaternary ammonium chitosan oligosaccharide: the N-methylene-O-quaternary ammonium chitosan oligosaccharide prepared in step (2) was added to water to make modifi...
Embodiment 1
[0053] (1) Preparation of N-methylene chitooligosaccharides
[0054] Dissolve 4 g of chitosan oligosaccharide in 50 mL of deionized water, take 9.3 mL of formaldehyde solution with a concentration of 15%, add it dropwise to the above solution, and stir for 4 h. 4 times the volume of ethanol was settled, filtered with suction, washed twice with ethanol, and vacuum-dried at 40°C until constant weight.
[0055] (2) Preparation of N-methylene O-quaternary ammonium chitosan oligosaccharide
[0056] Weigh 2g of the dried product in (1) and fully swell in 30mL of isopropanol, add 10mL of 40% NaOH solution, after continuing to swell, add 1.32g of 3-chloro-2-hydroxypropyltrimethyl chloride at 80°C Ammonium chloride was stirred for 24h. Adjust the pH to neutral, settle with 4 times the volume of acetone, filter with suction, wash with 80wt.%, 90wt.% and absolute ethanol in turn, and dry in vacuum at 40°C until constant weight.
[0057] (3) Synthesis of N-thiosemicarbazide-O-quaternar...
Embodiment 2
[0060] (1) Preparation of N-methylene chitooligosaccharides
[0061] Dissolve 4 g of chitosan oligosaccharide in 50 mL of deionized water, take 9.5 mL of formaldehyde solution with a concentration of 15%, add it dropwise to the above solution, and stir for 4 h. 4 times the volume of ethanol was settled, filtered with suction, washed twice with ethanol, and vacuum-dried at 40°C until constant weight.
[0062] (2) Preparation of N-methylene-O-quaternary ammonium chitosan oligosaccharide
[0063] Weigh 2g of the dried product in (1) and fully swell in 30mL of isopropanol, add 10mL of 40% NaOH solution, after continuing to swell, add 1.32g of 3-chloro-2-hydroxypropyltrimethyl chloride at 80°C Ammonium chloride was stirred for 24h. Adjust the pH to neutral, settle with 4 times the volume of acetone, filter with suction, wash with 80wt.%, 90wt.% and absolute ethanol in turn, and dry in vacuum at 40°C until constant weight.
[0064] (3) Synthesis of N-thiosemicarbazide-O-quaternar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com