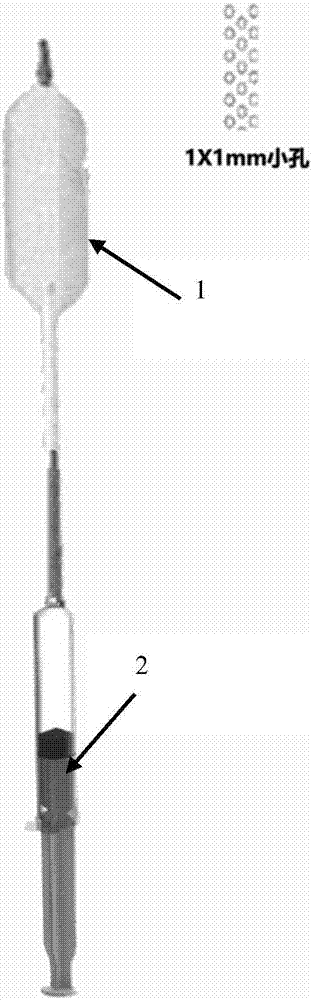
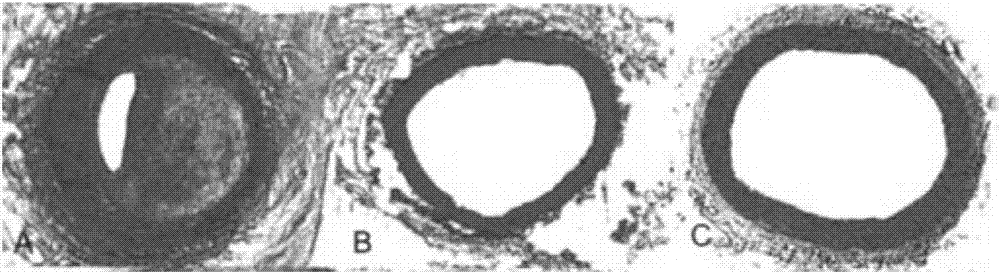
Coronary artery bypass grafting vascular grafts restenosis prevention system
A coronary artery and transplantation technique, applied in the field of medical devices, can solve the problems of inability to suppress long-term graft atherosclerosis, venous graft stenosis, and hemorrhagic complications, so as to improve the long-term patency rate and inhibit thrombosis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This system is composed of self-made paclitaxel-coated balloon with side holes and PLGA particles loaded with fluvastatin. The contact of endothelial cells exerts its cytotoxic effect and inhibits the proliferation of endothelial cells. Due to the filling pressure of the balloon, PLGA particles are pressed into the intercellular space, slowly releasing the drug ingredients, and inhibiting long-term atherosclerosis.
[0040] 1. Preparation of PLGA nanospheres
[0041] Use polylactic acid-polyglycolic acid copolymer (PLGA, 50:50, intrinsic viscosity 1.13dl / g) (commercially available, Xi'an Ruixi Biotechnology Co., Ltd.), the diameter of this nanoparticle is 10-500nm, and its polymer matrix can be The slow release of the drug is controlled, and its ultra-small size makes it very easy to enter the tissue space and reside locally in the tissue. Paclitaxel and fluvastatin are used to make a compound drug, which can simultaneously inhibit postoperative vascular endothelial hy...
Embodiment 2
[0049] In this embodiment, the size of the balloon is 5cm×25mm (length×diameter). The preparation method is the same as in Example 1.
Embodiment 3
[0051] In this embodiment, the size of the balloon is 5cm×30mm (length×diameter). The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com