A warfarin sodium orally disintegrating tablet capable of individual administration prepared by 3D printing and its preparation method
A technology of orally disintegrating tablets and warfarin sodium, applied in the field of preparation of the warfarin sodium orally disintegrating tablets, can solve problems such as narrow therapeutic window, large individual differences in curative effect, and failure to satisfy patients, and achieve drug release speed Fast, low drug side effects, and improved compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (Target dose: 2.50mg)
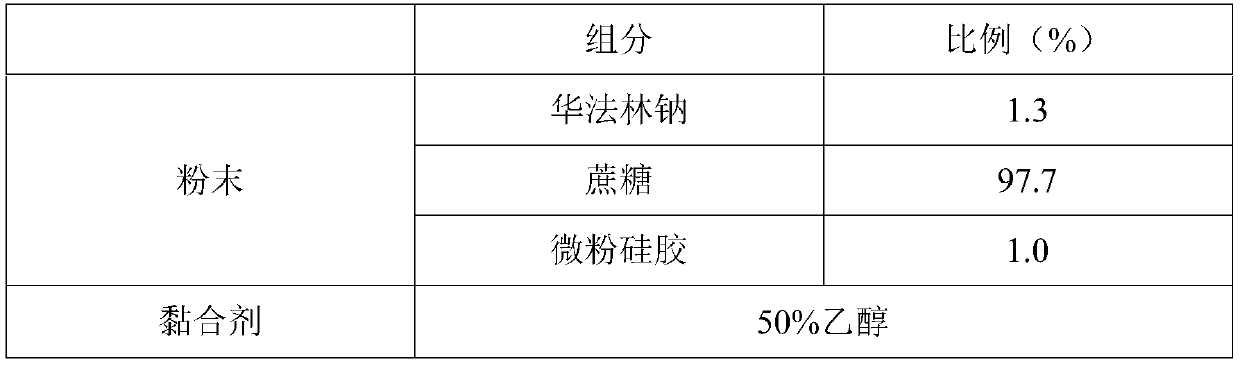
[0030] The formula is as follows:
[0031]
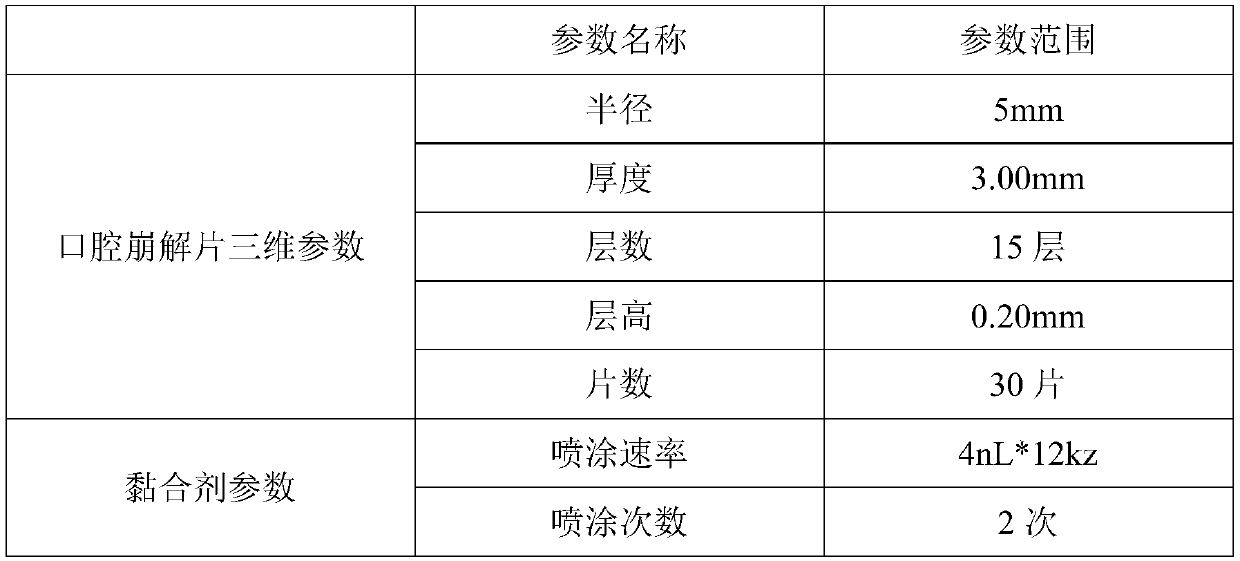
[0032] 3D printing parameters are as follows:
[0033]
[0034] The preparation method is as follows:
[0035] (1) According to the pre-set parameters, use CAD and other software to model, and convert it into an stl format file and import it into the LTY software control system of the 3D printer.
[0036] (2) Mix warfarin sodium, sucrose and micropowdered silica gel evenly, and put it into the powder box of the 3D printing rapid prototyping machine as a medicine powder.
[0037] (3) Put 50% ethanol solution into the cartridge.
[0038] (4) The control system outputs instructions to control the 3D printing rapid prototyping machine to print warfarin sodium orally disintegrating tablets: the powder spreading device first spreads a layer of powder with a thickness of 0.20mm on the powder bed, and then prints the nozzle in the direction of the X-Y axis. Move and spray the adhesive in a fixed area...
Embodiment 2
[0045] (Target dose: 3.00mg)
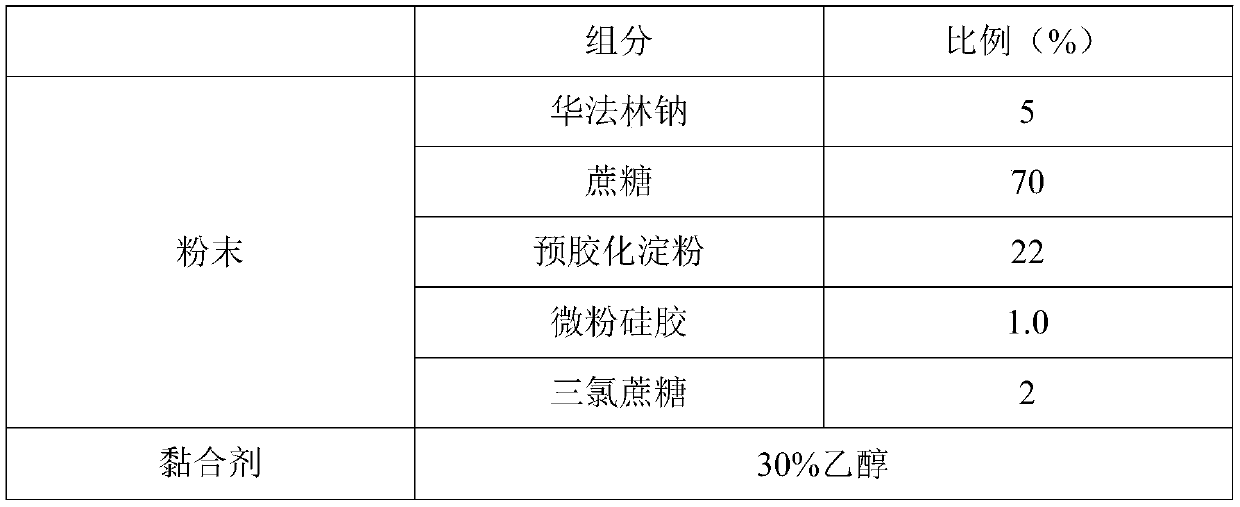
[0046] The preparation method and raw material ratio are the same as in Example 1, except that the number of printing layers is 18 and the thickness is 3.60mm. The quality inspection of warfarin sodium orally disintegrating tablets is as follows:
[0047] The content of warfarin sodium in the warfarin sodium orally disintegrating tablets determined by high performance liquid chromatography (HPLC) was 2.85 ± 0.08 mg / tablet.
[0048] Disintegration time limit test of warfarin sodium orally disintegrating tablets:
[0049] According to the "Chinese Pharmacopoeia" 2015 edition disintegration time determination method, the disintegration time limit of warfarin sodium orally disintegrating tablets was determined. The disintegration time is 8±2s, meeting the requirements of the 2015 edition of the Chinese Pharmacopoeia.
Embodiment 3
[0051] (Target dose: 5.00mg)
[0052] The preparation method and raw material ratio are the same as in Example 1, except that the number of printing layers is 30 and the thickness is 6.00mm. The quality inspection of warfarin sodium orally disintegrating tablets is as follows:
[0053] The content of warfarin sodium in the warfarin sodium orally disintegrating tablets determined by high performance liquid chromatography (HPLC) was 5.37 ± 0.34 mg / tablet.
[0054] Disintegration time limit test of warfarin sodium orally disintegrating tablets:
[0055] According to the "Chinese Pharmacopoeia" 2015 edition disintegration time determination method, the disintegration time limit of warfarin sodium orally disintegrating tablets was determined. The disintegration time is 10±2s, meeting the requirements of the 2015 edition of the Chinese Pharmacopoeia.
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com