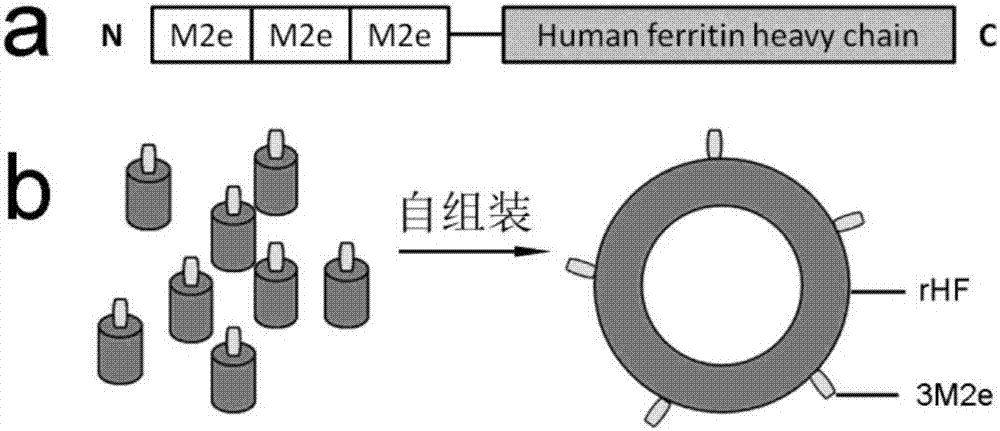
Nano influenza vaccine, construction method and application
A nano and recombinant protein technology, applied in chemical instruments and methods, biochemical equipment and methods, pharmaceutical formulations, etc., can solve problems such as difficulty in timely production of vaccines, and achieve simplified vaccine preparation process, improved immunogenicity, and less damage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
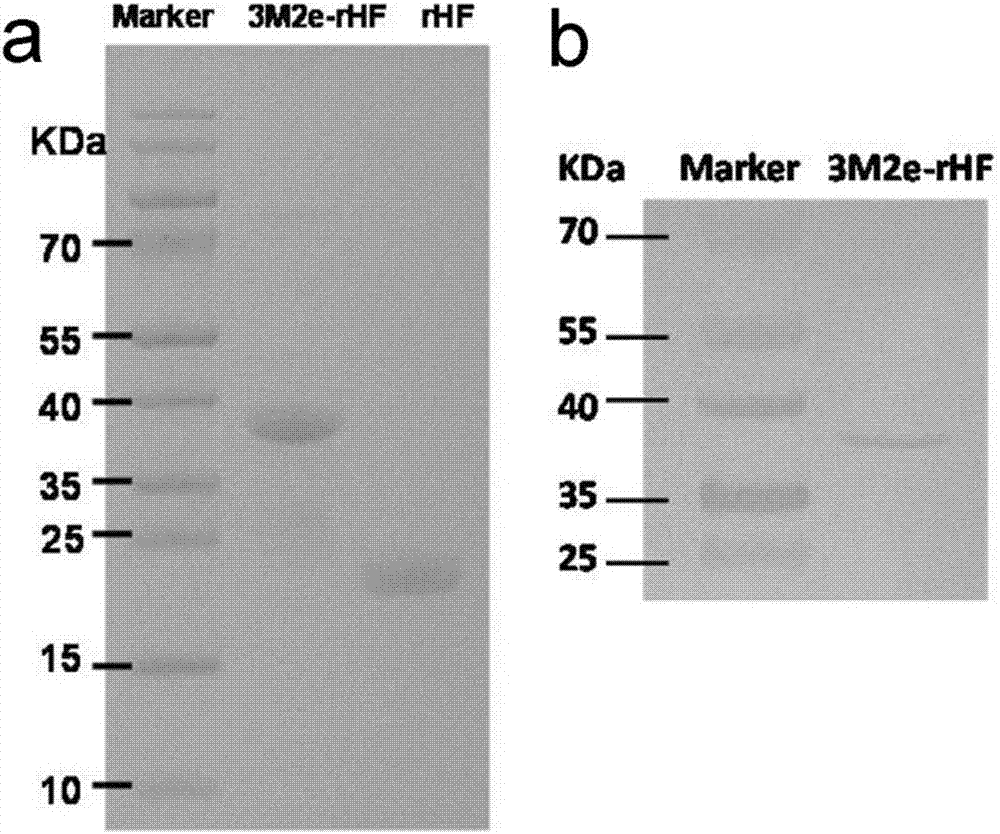
[0036] The preparation method of 3M2e-rHF nano flu vaccine, its steps are:
[0037] (1) Construct the 3M2e-rHF protein expression plasmid pET28a / 3M2e-rHF:
[0038] PCR amplification of rHF sequence: using the plasmid pET-rHF encoding human ferritin H chain sequence (rHF) (gifted by Dr PaoloSantambrogio (Milan, Italy)) as a template (Nanoscale, 2012, 4, 188), sense primer: 5'CCGGTGTAATGGAAGCTCCGACGGAGGAGGAGGATCTATGACGACCGCGTCCACCTC 3 ', reverse primer: 5' CCGCTCGAGTTAGCTTTTCATTATCACTGTC 3';
[0039] 3M2e sequence: The nucleic acid sequence of M2e (Gene ID: 956528) was found in NCBI, and the 3M2e sequence was chemically synthesized. Using the synthetic 3M2e nucleic acid sequence as a template, sense primer: 5'GGGAATTCCATATGATGTCTCTGCTGACCGAGG 3', reverse primer: 5'GAGGTGGACGCGGTCGTCATAGATCCTCCTCCTCCGTCGGAGCTTCCATTACACC 3', the amino acid sequence of M2e is MSLLTEVETPIRNEWGCRCNGSSD, and the sequence of influenza virus in A / Puerto Rico / 8 / 34 (H1N2e) unanimous.
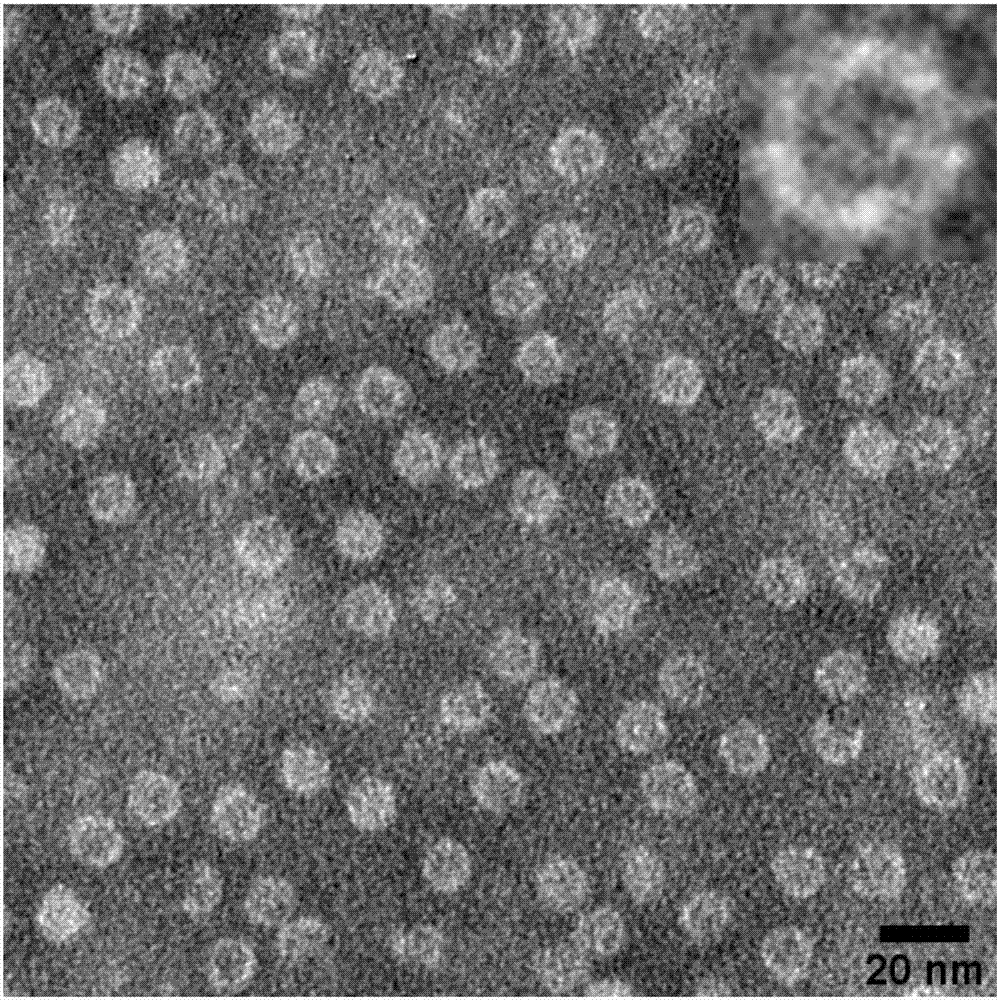
[0040]Using the ...
Embodiment 2
[0044] The verification of the immune effect of 3M2e-rHF nanoparticle influenza vaccine in mice, the steps are as follows:
[0045] (1) Balb / c mice aged 6 to 8 weeks were randomly divided into groups, and 10 μg of 3M2e-rHF nanoparticles, 2.6 μg of 3M2e synthetic peptides (compared with 3M2e-rHF nanoparticles containing equimolar M2e), Balb / c mice were immunized with 6.3 μg of rHF nanoparticles (compared with 3M2e-rHF nanoparticles containing equimolar rHF) and PBS via intranasal route for three times, with an interval of three days between two adjacent immunizations. week. Mouse serum was collected two weeks after each immunization, stored at -20°C, and ELISA was used to detect M2e-specific antibodies. Antibody detection results showed that after three immunizations, the 3M2e polypeptide and rHF nanoparticles immunized mice were the same as the PBS control mice, and M2e-specific IgG antibodies were not detected in the serum, while 3M2e-rHF nanoparticles induced a large amount...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com