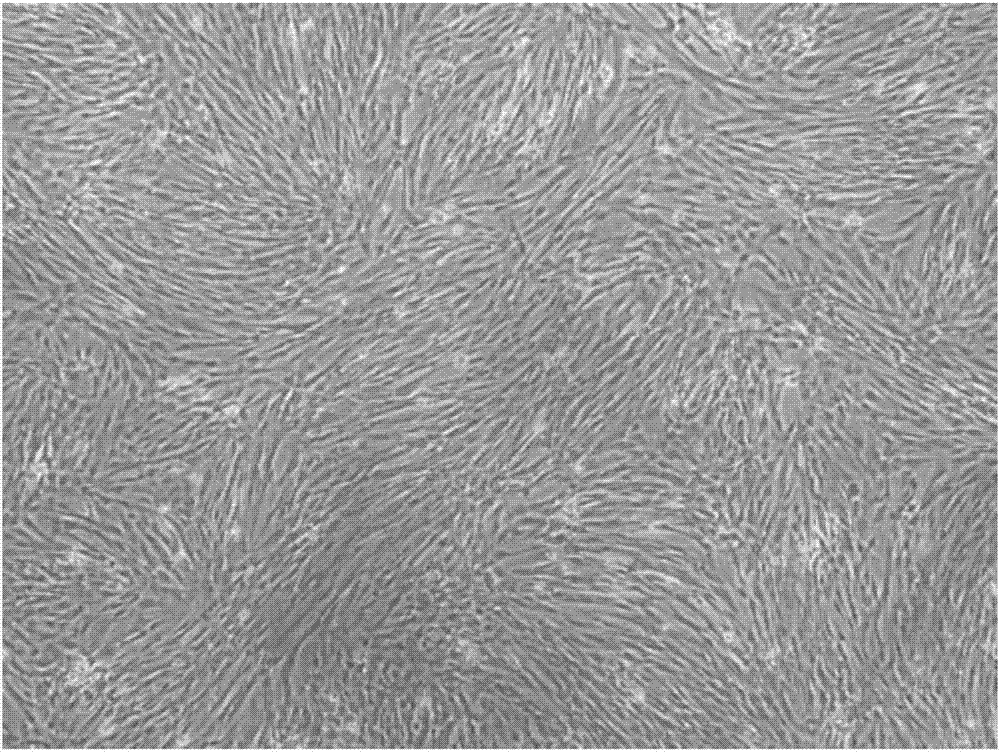
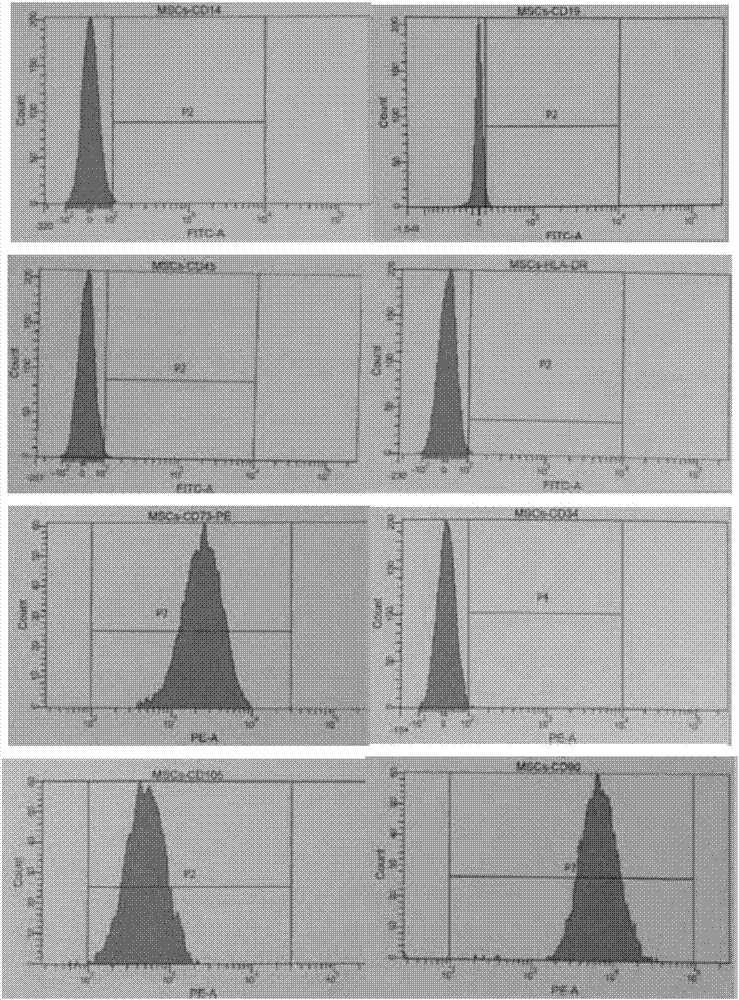
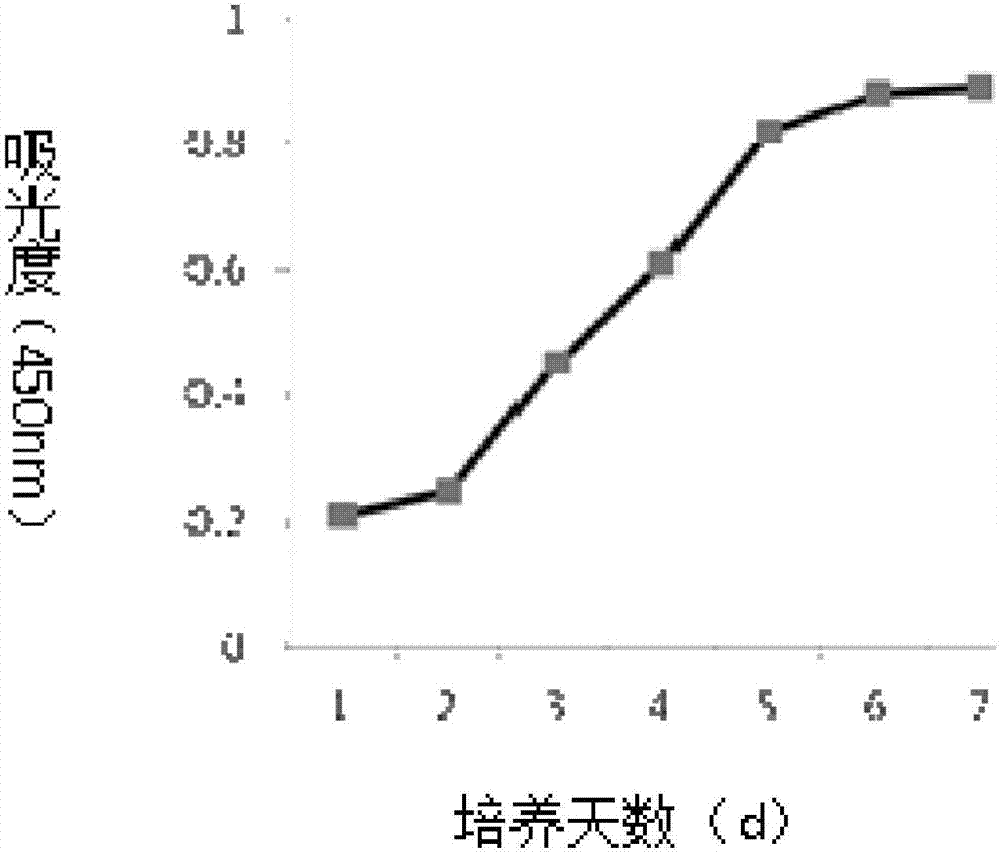
Human placenta chorionic mesenchymal stem cell separation method
A technology of mesenchymal stem cells and separation methods, applied in the field of human placental chorionic mesenchymal stem cells, can solve the problems of decreased differentiation ability and cell number, strong immune rejection of allogeneic transplantation, and high chance of virus infection, etc., to reduce manual processing time and manpower consumption, the effect of increasing the area of tissue adhering to the wall and reducing the chance of contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1) Human placental chorionic tissue was taken, cut into 1-3 cm strips, and washed repeatedly with normal saline until there was no blood;
[0048] 2) Use a hand-held electric homogenizer to process the chorion to 0.1-0.3cm 3 For fine particles, wash again with normal saline until clear;
[0049] 3) Process again with a homogenizer to 0.1-0.5mm 3 After left and right small particles, centrifuge at 300r / min for 5min to remove the upper layer of blood cells;
[0050] 4) Add the same volume of trypsin and collagenase II to the precipitation, and digest at 37°C for 0.5h;
[0051] 5) After the digest was centrifuged and washed twice with normal saline, the culture medium was added to the precipitate and placed at 37°C, 5% CO 2 Cultivated in an incubator;
[0052] 6) Discard the tissue pieces and culture medium on the 7th day, rinse the bottom of the culture bottle with normal saline, add fresh medium, and then replace the medium every 3 days until the cell confluence reac...
Embodiment 2
[0054] 1) Human placental chorionic tissue was taken, cut into 1-3 cm strips, and washed repeatedly with normal saline until there was no blood;
[0055] 2) Use a hand-held electric homogenizer to process the chorion to 0.1-0.3cm 3 For fine particles, wash again with normal saline until clear;
[0056] 3) Process again with a homogenizer to 0.1-0.5mm 3 After left and right small particles, centrifuge at 500r / min for 5min to remove the upper layer of blood cells;
[0057] 4) Add the same volume of trypsin and collagenase II to the precipitate, and digest at 37°C for 1 hour;
[0058] 5) After the digest was centrifuged and washed twice with normal saline, the culture medium was added to the precipitate and placed at 37°C, 5% CO 2 Cultivated in an incubator;
[0059] 6) Discard the tissue pieces and culture medium on the 7th day, rinse the bottom of the culture bottle with normal saline, add fresh medium, and then replace the medium every 3 days until the cell confluence reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com