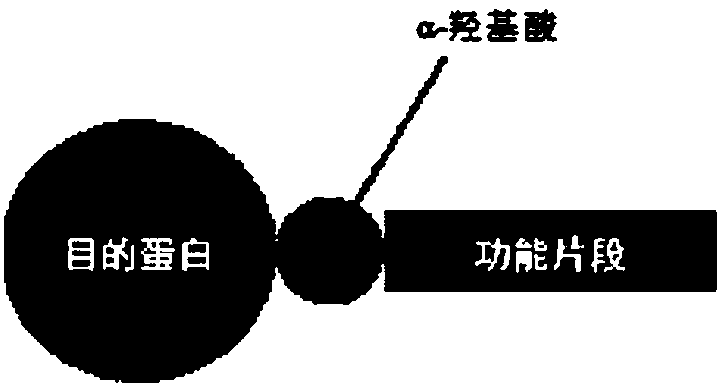
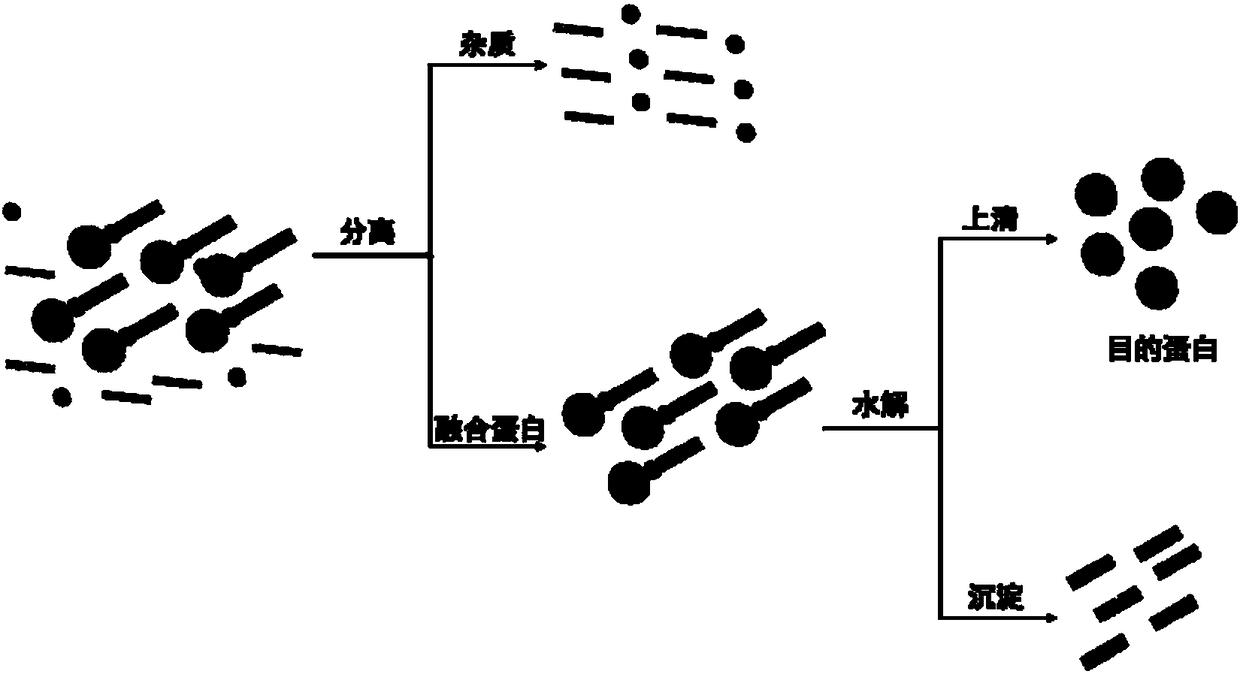
α-Hydroxy Acid-Based Protein Purification Methods
A protein purification and hydroxy acid technology, which is applied in the direction of peptide preparation methods, chemical instruments and methods, biochemical equipment and methods, etc., can solve problems such as inapplicability, improve purification efficiency and yield, overcome high costs, reduce The effect of time and economic cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Example 1 Expression and purification of fusion protein using wild-type defensin 5 (Defensin 5) as the target protein
[0119] The solutions and medium components used in Example 1 are as follows.
[0120] 1x PBS buffer:
[0121] NaCl: 7.9g (Beijing Chemical Plant, purity ≥99.5%, pH: 5.0-8.0); KCl: 0.2g (Beijing Chemical Plant, purity ≥99.5%, pH: 5.0-8.0);
[0122] K H 2 PO 4 : 0.24g (Beijing Chemical Plant, purity ≥ 99.5%, pH: 4.2 ~ 4.5);
[0123] K 2 HPO 4 : 1.8g (Beijing Chemical Plant, purity ≥ 99.0%, pH: 8.9 ~ 9.4);
[0124] Dissolve in 800ml of distilled water, and finally add distilled water to make up to 1L, and adjust the pH value of the solution to 7.4 with HCl (analytical grade, Sinopharm Chemical Reagent Co., Ltd.).
[0125] Luria-Bertani (LB) liquid medium:
[0126] Peptone (Fisher Scientific) 10g / L;
[0127] NaCl (Fisher Scientific) 10g / L;
[0128] Yeast powder (Fisher Scientific) 5g / L;
[0129] pH=7.
[0130] Autoclave at 121°C for 20 minutes....
Embodiment 2
[0166] Example 2 Expression and purification of fusion protein using wild-type histidine-rich peptide (Histatin) as target protein
[0167] Unless otherwise specified, the buffer solution, culture medium, enzyme, initial plasmid and assay method used in Example 2 are all the same as in Example 1.
[0168] LB liquid and solid media were used in plasmid construction, strain cultivation, maintenance, and induction.
[0169] 1. Construction of expression strains
[0170] In this example, the wild-type histidine-rich peptide (Histatin) (GenBank: NM_002159.3) was used as the target protein. Hisstatin has antibacterial activity. The activity of the protein can be visually detected by dropping it on the surface of a plate coated with bacteria and observing the size of the inhibition zone.
[0171] In this example, ABZ (SEQ ID NO: 2) with self-assembly function is used as a functional fragment. Since ABZ has self-assembly function, it can aggregate in the expression cell to form insol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com