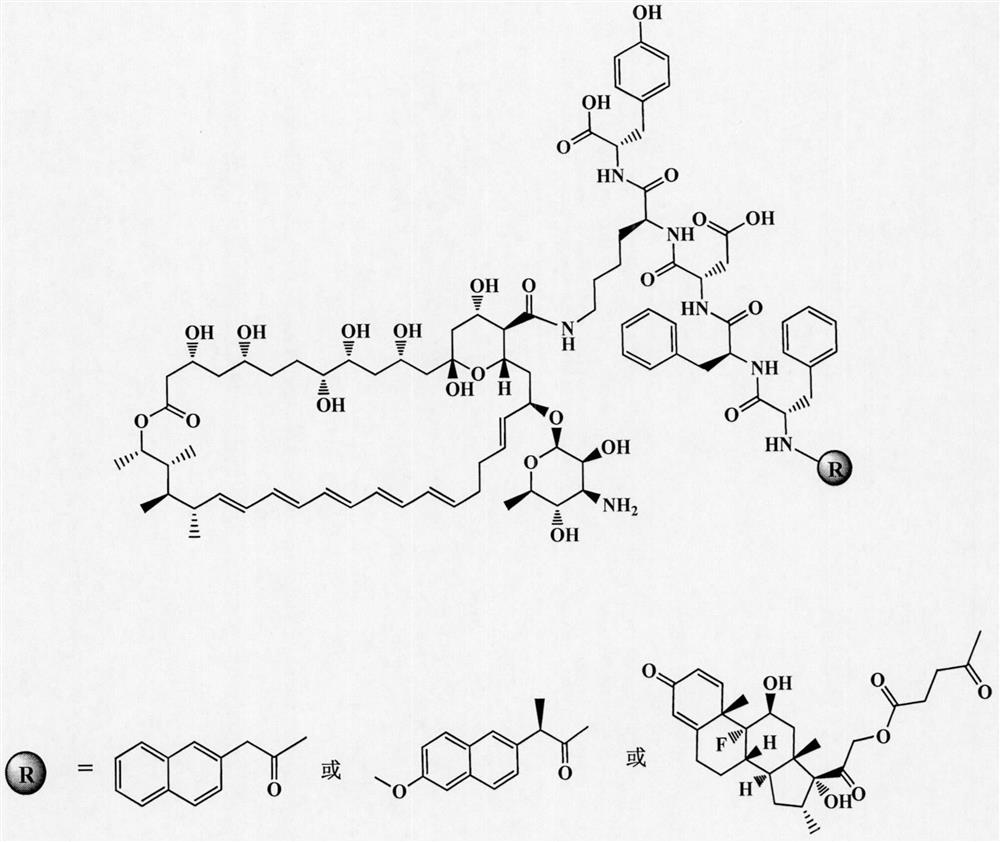
Amphotericin b polypeptide hydrogel drug-loading system for the treatment of fungal infections
A technology for amphotericin and fungal infection, applied in antifungal agents, dipeptide components, pharmaceutical formulations, etc., can solve the problems of low biocompatibility, clinical toxicity and side effects of bioavailability, improve the transport efficiency in vivo, improve Biocompatibility, the effect of reducing clinical toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh about 1.2g of 2-chlorotrityl chloride resin (1.0~1.2mmol / g) into the solid-phase synthesis tube, add 15mL of dichloromethane (DCM for short), swell for 20 minutes, filter, wash with DCM for 2min× 5 times. Add the first amino acid, 15mL DCM and N,N-diisopropylethylamine (DIPEA for short) to the above-mentioned solid-phase synthesis tube according to the dosage in Table 1, shake for 2 hours and drain, and wash with DCM for 2min×5 times. Add 10 mL of DCM / methanol / DIPEA (80:15:5) mixture to block the activated sites of the resin, shake for 15 min x 2 times, then drain, and wash with N,N-dimethylformamide (DMF) for 2 min x 5 times. Add 10 mL of 20% piperidine / DMF (V / V) solution and shake for 5 minutes to drain, add 10 mL of this solution and shake for 30 minutes, wash with DMF for 2 minutes x 5 times.
[0035] Add the second amino acid, 2-(7-azobenzotriazole)-tetramethyluronium hexafluorophosphate (referred to as HBTU) and 1-hydroxybenzotriazole (referred to as HOBT),...
Embodiment 2
[0040] According to the Nap-FFDKY compound prepared in Example 1, weigh 132.9 mg of the Nap-FFDKY compound and 92.41 mg of amphotericin B and dissolve them completely in 5 ml of DMF, add 41.25 μL of DIPEA, stir overnight in the dark, add glacial ether to disperse and extract, The solid was obtained by centrifugation, and was purified by HPLC after adding DMF to dissolve, and the purification conditions were as follows: the chromatographic column adopts Phenomenex Luna C 18 (50×300 mm, 10 μm), the mobile phase is methanol:water (55:45, V / V), the detection wavelength is 280 nm, and the flow rate is 15 ml / min. After preparation, the components were combined, concentrated by rotary evaporation, and freeze-dried to obtain the pure Nap-AmB product with a yield of about 69.1%.
Embodiment 3
[0042] According to the Npx-FFDKY compound prepared in Example 1, weigh 139.6 mg Npx-FFDKY and 92.41 mg amphotericin B and dissolve them completely in 5 ml DMF, add 41.25 μL DIPEA, stir overnight in the dark, add glacial ether to disperse and extract, and separate The obtained solid was purified by HPLC after adding DMF for dissolution, and the purification conditions were as follows: the chromatographic column adopts Phenomenex Luna C 18 (50×300 mm, 10 μm), the mobile phase is methanol:water (45:55, V / V), the detection wavelength is 280 nm, and the flow rate is 15 ml / min. After preparation, the components were combined, concentrated by rotary evaporation, and freeze-dried to obtain pure Npx-AmB with a yield of about 53.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com