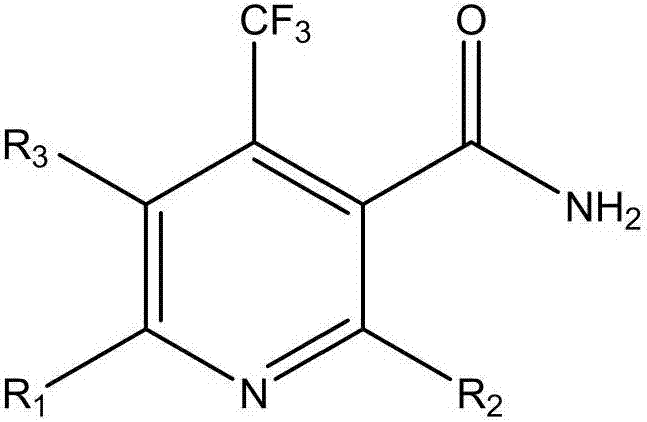
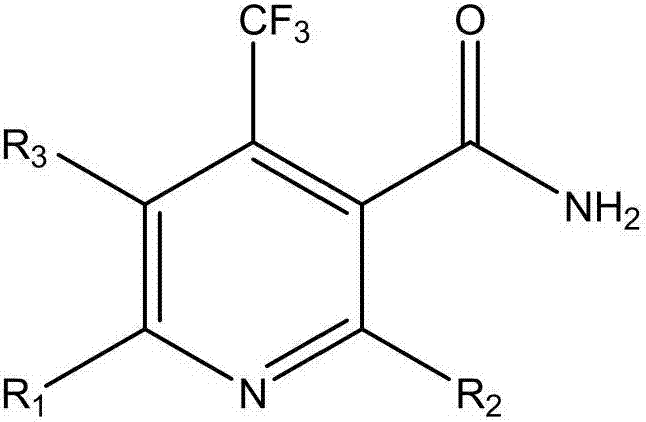
Nicotinamide compound as well as preparation method and application thereof
A compound, the technology of nicotinamide, applied in the field of nicotinamide compound and its preparation, can solve the problems of environmental pollution, plant drug resistance, irrational use of chemical pesticides, etc., and achieve a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the route one comprises the following steps:
[0028] A, potassium hydroxide is added in the flask under stirring state, then slowly add methanol solution, form potassium hydroxide methanol solution after stirring and cooling, stand for use;
[0029] B. Add cyanoacetamide to the four-neck flask under stirring, then add methanol solution, stir and heat to reflux, the reaction solution turns from turbid to transparent, and then add the raw material ethyl trifluoroacetoacetate, in which cyanoacetamide and trifluoroacetoacetate The molar ratio of ethyl ester is 1:0.5~1.5, add potassium hydroxide methanol solution while maintaining the reflux state, continue to reflux for 24 hours, and wait for the complete reaction of cyanoacetamide to obtain the reflux solution;
[0030] C. Distill the reflux solution, recover 3 / 4 methanol, add cold water to the residue, under cooling condition, use dilute hydrochloric acid to adjust PH=3-4, after stirring for 0.5...
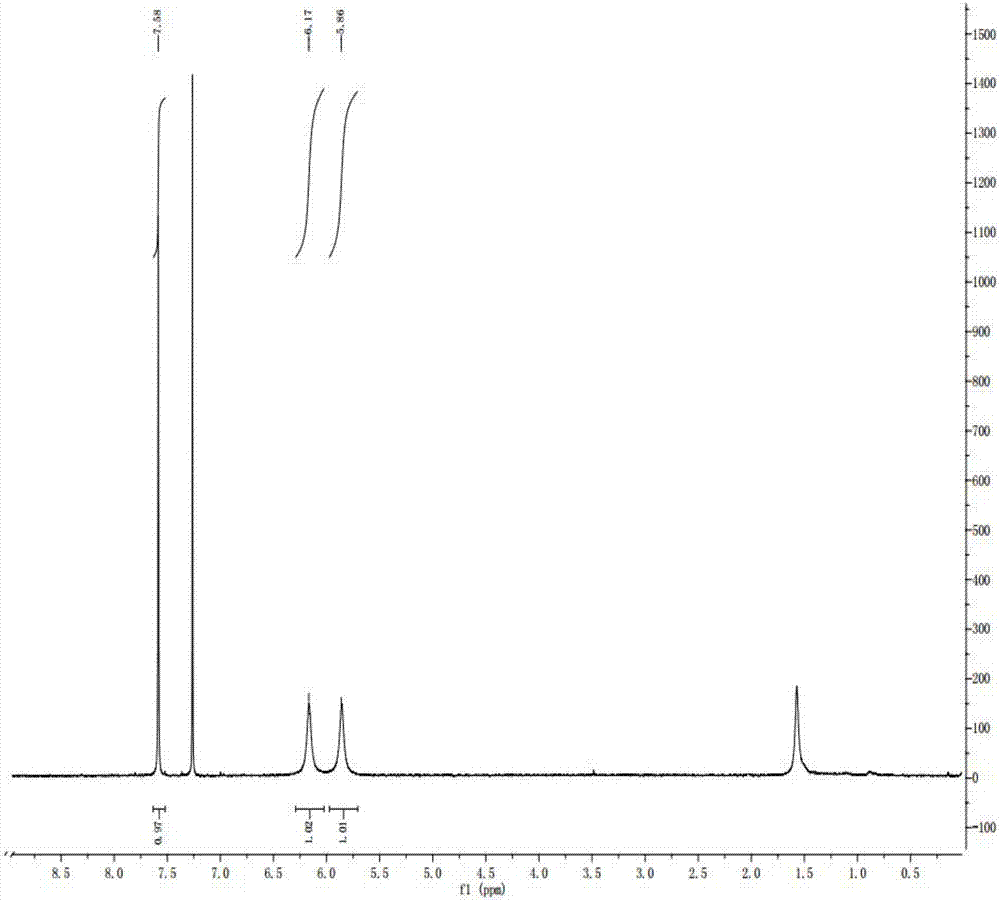
Embodiment 1
[0043] Example 1: Preparation of 2,6-dichloro-4-(trifluoromethyl)nicotinamide
[0044] (1) Preparation of intermediate 2,6-dihydroxy-3-cyano-4-(trifluoromethyl)pyridine
[0045] Take 29.8g (0.53mol) of potassium hydroxide and put it into a 250mL four-necked bottle equipped with mechanical stirring and reflux condenser, slowly add 100mL of methanol, stir and cool for later use; put 43.4g (0.52mol) of cyanoacetamide into a bottle equipped with mechanical stirring , constant pressure dropping funnel, drying tube and 500mL four-necked flask of reflux condenser, add 150mL of methanol, mechanically stir and heat to reflux, after the reaction solution changes from turbid to light yellow and transparent, add 100g (0.54mol) trifluoroacetoacetic acid Ethyl ester, keep the reflux state, slowly add the prepared potassium hydroxide methanol solution dropwise, heat and reflux for 24 hours, the reaction of the central control raw material cyanoacetamide is complete, change the reflux device ...
Embodiment 2
[0050] Example 2: Preparation of 2,6-difluoro-4-(trifluoromethyl)nicotinamide
[0051] (1) Preparation of intermediate 3-cyano-2,6-difluoro-4-(trifluoromethyl)pyridine
[0052] Add 53 grams of 2,6-dichloro-3-cyano-4-(trifluoromethyl)pyridine, 35 grams of KF, 10 grams of 18 crown ether, and 250 ml of DMF into a 250ml four-necked bottle, heat up to 130 degrees, and keep warm for 3 -5 hours, after the reaction is completed, add water to precipitate a yellow solid, 30 g after drying, hplc92%, yield 65.5%.
[0053] (2) Preparation of 2,6-difluoro-4-(trifluoromethyl)nicotinamide
[0054] Add 25 grams (120 mmol) of 3-cyano-2,6-difluoro-4-(trifluoromethyl)pyridine and 75 grams of 98% concentrated sulfuric acid into a 250ml four-necked bottle, heat up to 90-95 degrees, and keep warm for 3 hours After the temperature was lowered, it was added into water, and a pale yellow solid was precipitated. After drying, 23 g was obtained, hplc93%, and the yield was 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com