Method for cyclic compound ring opening polymerization
A ring-opening polymerization technology for cyclic compounds, which is applied in the field of organic catalysis and polymer materials, can solve the problems of few catalyst preparation methods, and achieve the effects of large commercial application potential, narrow molecular weight distribution, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
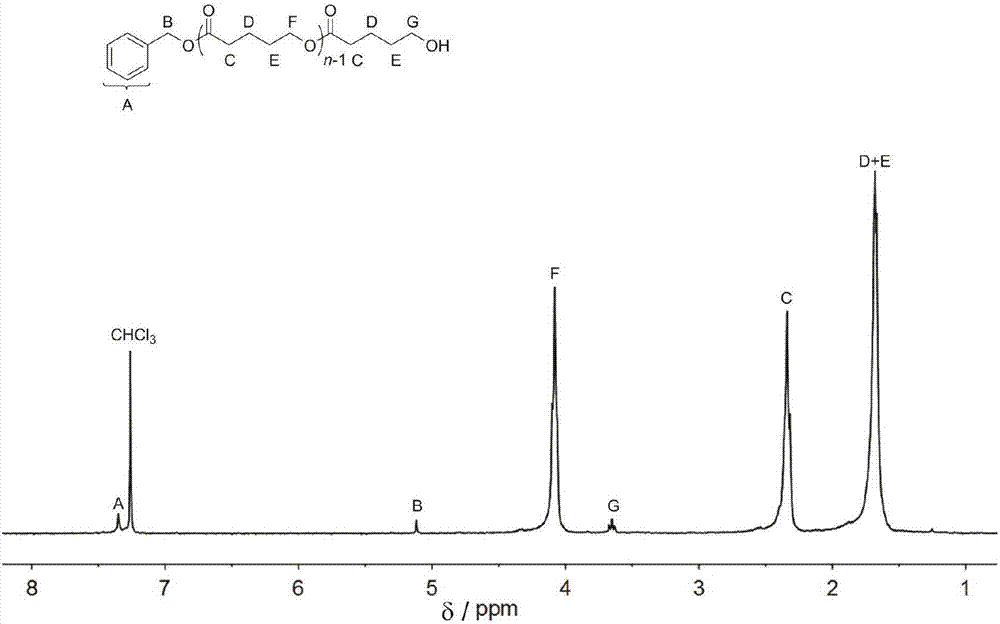
[0042] Add organic salt (1) (33.3mg, 0.1mmol, 1.0equiv), benzyl alcohol (10.3μL, 0.1mmol, 1.0equiv) and δ-valerolactone (0.27ml, 3.0mmol, 30equiv) into the reaction flask, and use 1 mL of dichloromethane was dissolved, and under the protection of Ar, the reaction was stirred at room temperature for 12 hours. The reactant was concentrated and poured into methanol, the precipitate was filtered and dried to constant weight, the conversion rate was 96% (proton nuclear magnetic resonance spectrum, 400MHz, CDCl 3 ), the number average molecular weight M of polyvalerolactone n 2850g mol -1 , the dispersion PDI is 1.08 (molecular exclusion chromatography, Waters column: 5mm, 300 7.8mm, tetrahydrofuran mobile phase, 0.7mL min-1, polystyrene as standard), 1 H NMR (400MHz, CDCl 3 ):δ(ppm)1.68(m,2H×n,(–CH2CH2CH2O–)n),1.70(m,2H×n,(–COCH2–CH2CH2–)n),2.34(t,2H×n,J= 6.8Hz, (–OCOCH2CH2–)n), 3.65(t, 2H, J=6.1Hz, –CH2CH2OH), 4.08(t, 2H×n, J=5.5Hz, (–CH2CH2O-)n), 5.12(s , 2H, ArCH2O), 7.32...
Embodiment 2
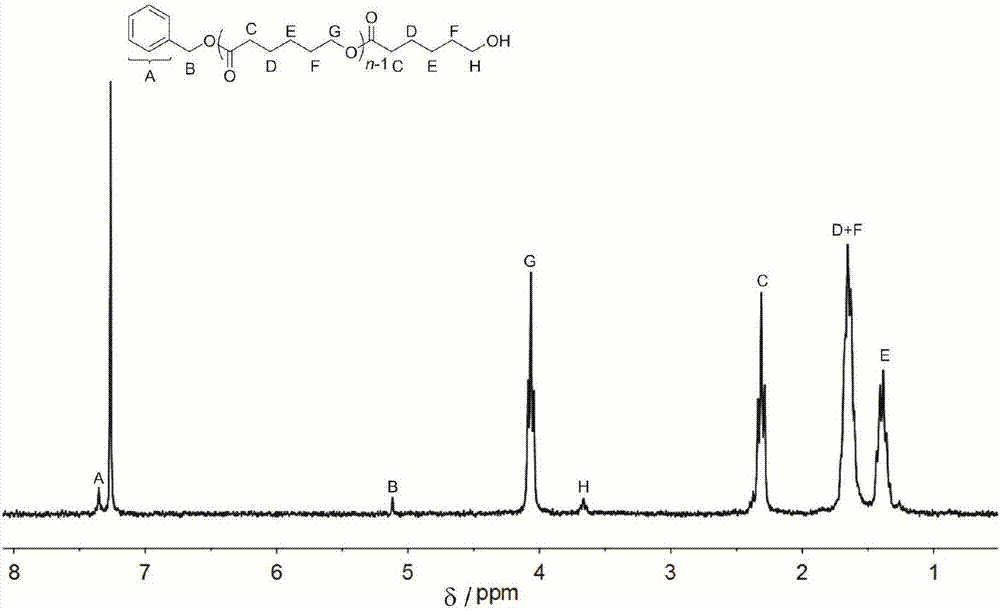
[0045] Organic salt (2) (22.1 mg, 0.05 mmol, 0.5 equiv), 5-hexen-1-ol (12.0 μL, 0.1 mmol, 1.0 equiv) and ε-caprolactone (5.5 ml, 50 mmol, 500 equiv) were added In the reaction flask, the temperature was raised to 180° C., and the reaction was stirred for 20 hours under the protection of Ar. The reaction solution was poured into ethanol, the precipitate was filtered and dried to constant weight, the conversion rate was 92% (proton nuclear magnetic resonance spectrum, 400MHz, CDCl 3 ), the number average molecular weight M of polycaprolactone n 41300g mol -1 , the dispersion PDI is 1.27 (molecular exclusion chromatography, Waters column: 5mm, 300 7.8mm, tetrahydrofuran mobile phase, 0.7mL min-1, polystyrene as the standard sample); 1 H NMR (400MHz, CDCl 3 ):δ(ppm),1.39(m,2H×n,(–CH2CH2CH2CH2CH2–)n),1.63(m,2H×n,(–CH2CH2CH2O–)n),1.68(m,2H×n,(–COCH2CH2CH2 –)n), 2.31(t, 2H×n, J=7.3Hz, (–OCOCH2CH2–)n), 3.65(t, 2H, J=6.6Hz, CH2CH2OH), 4.06(t, 2H×n, J= 6.6Hz, (–CH2CH2O–)n), 5.12(s,...
Embodiment 3
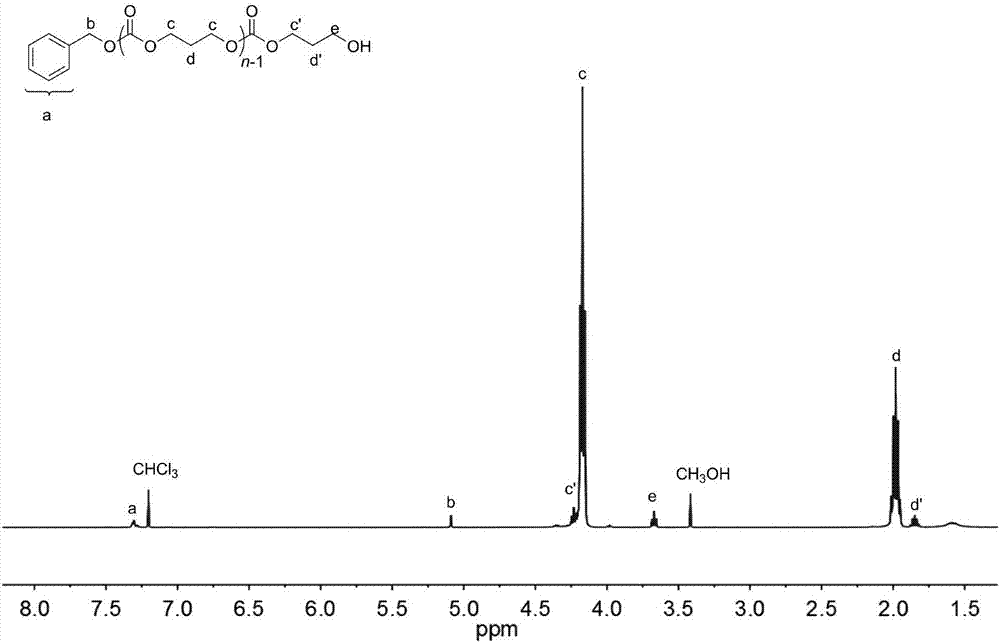
[0048] Add organic salt (4) (1955mg, 3mmol, 30equiv), propynyl alcohol (5.8μL, 0.1mmol, 1.0equiv) and carbonate (0.3mg, 3.0mmol, 30equiv) into the reaction flask, dissolve with 5mL of chloroform, Under the protection of Ar, the reaction was stirred at room temperature for 24 hours. The reactant was concentrated and poured into n-hexane, the precipitate was filtered and dried to constant weight, the conversion rate was 92% (proton nuclear magnetic resonance spectrum, 400MHz, CDCl 3 ), the number average molecular weight M of polycarbonate n 2820g mol -1 ;, the dispersion PDI is 1.10 (size exclusion chromatography, Waters column: 5mm, 300 7.8mm, tetrahydrofuran mobile phase, 0.7mL min-1, polystyrene as standard); 1 H NMR (400MHz, CDCl 3 ):δ(ppm)1.91(q,2H,J=6.1Hz,–CH2CH2OH),2.02–2.07(m,2H×n-1,(–OCH2CH2–)n-1),3.73(t,2H,J =6.0Hz, –CH2OH), 4.22–4.30(m, 4H×n-1, (–OCH2CH2CH2O–)n-1; m, 2H, –OCH2CH2CH2OH), 5.15(s, 2H, ArCH2O), 7.25–7.37( m, 5H, aromatic).
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com