Method for preparing vanadium trioxide from vanadium-containing solution
A vanadium trioxide solution technology, applied in the field of vanadium chemical industry, can solve the problems of low cost, difficult reduction reaction, short process flow, etc., and achieve low production cost, reduced reaction temperature and partial pressure of hydrogen, and short process flow Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
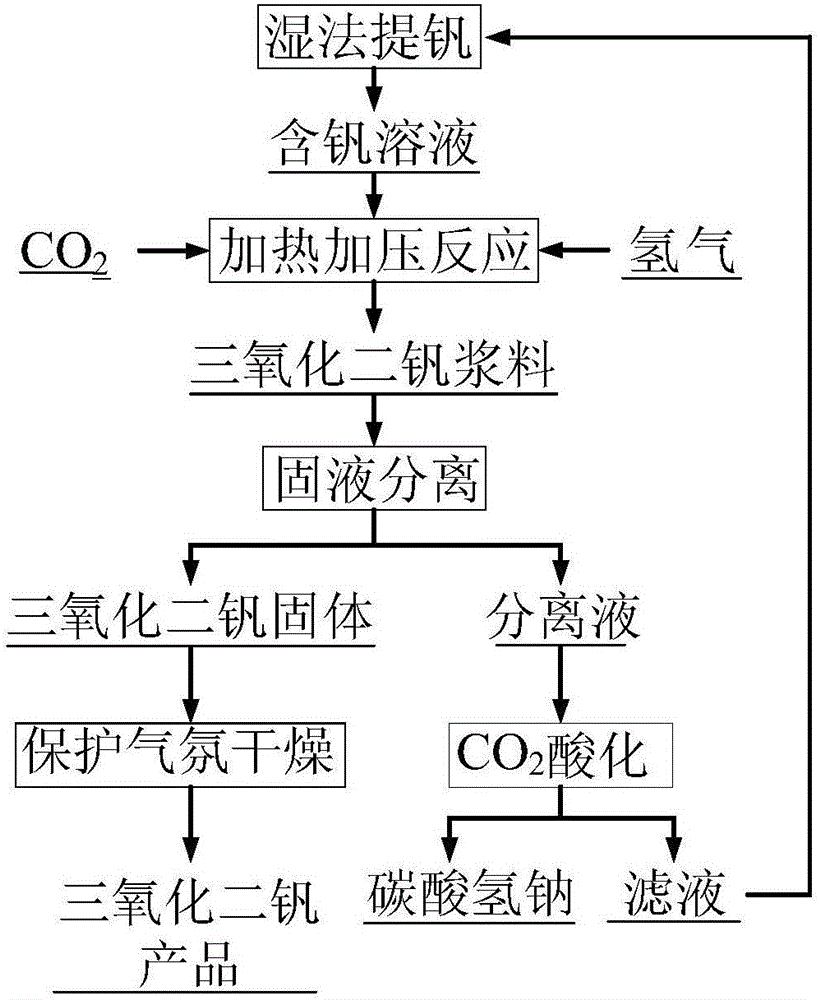
[0052] (1) Pass CO into the sodium pyrovanadate solution containing vanadium concentration of 46g / L 2 and H 2 , at 120°C, CO 2 Partial pressure 0.5MPa, H 2 Under the conditions of partial pressure 2MPa and stirring speed of 500r / min, react in a high-temperature and high-pressure reactor for 2 hours, filter after the reaction is completed, and obtain vanadium trioxide solid and separation liquid;
[0053] (2) drying the vanadium trioxide solid obtained in step (1) at 105° C. under a nitrogen atmosphere to obtain a vanadium trioxide product;
[0054] (3) At 15°C, feed CO into the separation liquid obtained in step (1) 2 Carry out acidification reaction, stir at a speed of 200r / min, the pH value of the reaction end point is 8.0, filter and separate sodium bicarbonate crystals and filtrate after reacting for 1.5h; return the obtained filtrate to the sodium vanadate solution preparation process for recycling.
[0055] After testing, the reduction rate of vanadate in this embodi...
Embodiment 2
[0057] (1) Pass CO into the sodium pyrometamate solution containing vanadium concentration of 32g / L 2 and H 2 , at 150°C, CO 2 Partial pressure 1MPa, H 2 Under the condition of partial pressure of 2.5MPa and stirring speed of 700r / min, react in a high-temperature and high-pressure reactor for 3 hours, filter after the reaction is completed, and obtain vanadium trioxide solid and separation liquid;
[0058] (2) drying the vanadium trioxide solid obtained in step (1) at 280° C. under a nitrogen atmosphere to obtain a vanadium trioxide product;
[0059] (3) At 25°C, feed CO into the separation liquid obtained in step (1) 2 Perform acidification reaction, stir at a speed of 250r / min, the pH value of the reaction end point is 8.2, filter and separate sodium bicarbonate crystals and filtrate after reacting for 2 hours; return the obtained filtrate to the sodium pyrovanadate solution preparation process for recycling.
[0060] After testing, the reduction rate of vanadate in this...
Embodiment 3
[0062] (1) To the vanadium concentration of 25g / L containing sodium metavanadate solution into the CO 2 and H 2 , at 100°C, CO 2 Partial pressure 0.8MPa, H 2 Under the condition of partial pressure of 2.2MPa and stirring speed of 350r / min, react in a high-temperature and high-pressure reactor for 3 hours, and filter after completion of the reaction to obtain vanadium trioxide solid and separation liquid;
[0063] (2) drying the vanadium trioxide solid obtained in step (1) at 200° C. under an argon atmosphere to obtain a vanadium trioxide product;
[0064] (3) At 20°C, feed CO into the separation liquid obtained in step (1) 2 Carry out acidification reaction, stir at a speed of 100r / min, the pH value of the reaction end point is 7.8, filter and separate sodium bicarbonate crystals and filtrate after reacting for 1 hour; return the obtained filtrate to the sodium metavanadate solution preparation process for recycling.
[0065] After testing, the reduction rate of vanadate i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com