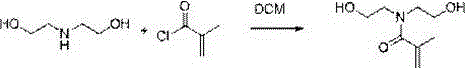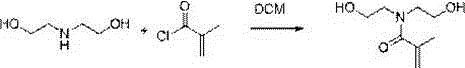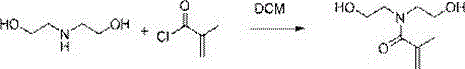Preparation method of N,N-bis(2-hydroxyethyl)methacrylamide
A technology of methacrylamide and hydroxyethyl, applied in the N field, can solve the problems of low yield of synthesis process, difficult purification, poor economic benefits, etc., and achieves the advantages of good process reproducibility, simple operation and reduced production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The first step: the synthesis of 2,2-dimethyl-1,3-dioxo-6-azacyclooctane.
[0037] Diethanol ammonia (5 kg, 47.58 mol, 1.00 eq) was added to 22 kg of methanol, and hydrogen chloride (1.02 eq) was passed through at 0-10 °C. The resulting solution was stirred at room temperature for 1 hour, 2,2-dimethoxypropane (27kg, 259mol, 5.4 eq) and p-toluenesulfonic acid (326 g, 1.90 mmol, 0.04 eq) were added, and the resulting solution was refluxed for reaction 3-4 Hour. Filter, wash the filter cake with acetone, add it to the dichloromethane solution, adjust the pH value to 9-10 with 2N aqueous sodium hydroxide solution, separate the organic phase, extract the aqueous phase twice with dichloromethane, and combine the organic phases with rotary evaporation 6.49 kg of pale yellow oil were obtained, with a yield of 94%.
[0038] After detection, the mass spectrometry data are as follows, and it is determined that the oily substance is 2,2-dimethyl-1,3-dioxo-6-azacyclooctane.
[00...
Embodiment 2
[0051] The first step: the synthesis of 2,2-dimethyl-1,3-dioxo-6-azacyclooctane.
[0052] Diethanolamine ( 5 kg, 47.58 mol, 1.00 eq) was added to 22 kg of methanol, and hydrogen bromide (1.02 eq) was introduced at 0-10 °C. The resulting solution was stirred at room temperature for 1 hour, 2,2-dimethoxypropane (34.7 kg, 333 mol, 7 eq) and p-toluenesulfonic acid (406 g, 2.37 mmol, 0.05 eq) were added to the resulting solution at 85°C Stir for 3-4 hours. Filter, wash the filter cake with acetone, dissolve it with dichloromethane, adjust the pH value to 9-10 with 2N aqueous sodium hydroxide solution, separate the organic phase, and extract the aqueous phase twice with dichloromethane. Dry with sodium sulfate, filter and spin dry to obtain 6.50kg of light yellow oil, the yield is 94%.
[0053] After detection, the mass spectrometry data are as follows, and it is determined that the oily substance is 2,2-dimethyl-1,3-dioxo-6-azacyclooctane.
[0054] ESI / MS: m / z=146.2[MH] +
[0...
Embodiment 3
[0065] The first step: the synthesis of 2,2-dimethyl-1,3-dioxo-6-azacyclooctane.
[0066] Diethanolamine (500 g, 4.76 mol, 1.00 eq) was added to dioxane (1.5 L), and hydrogen chloride (1.02 eq) was passed through at 0-10°C. The resulting solution was stirred at room temperature for 1 hour, and 2,2-dimethoxypropane (1.49 kg, 14.28 mol, 3 eq) and p-toluenesulfonic acid (24 g, 0.14 mmol, 0.03 eq) were added. The resulting solution was stirred at 85°C for 3-4 hours. After filtering, the filter cake was washed with acetone, added to dichloromethane solution, adjusted to pH 9 with 2N aqueous sodium hydroxide solution, the organic phase was separated, the aqueous phase was extracted twice with dichloromethane, and the organic phases were combined with anhydrous Dry over sodium sulfate, filter and spin dry to obtain 669 g of light yellow oil, with a yield of 97%.
[0067] After detection, the mass spectrometry data are as follows, and it is determined that the oily substance is 2,2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com