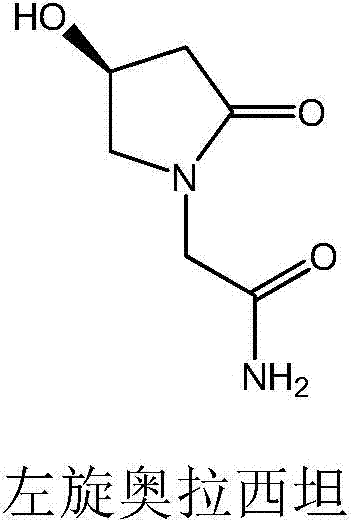
Levorotatory oxiracetam injection and preparation method thereof
A technology for injections and injections, which is applied in the field of levo-oxiracetam, can solve the problems that the clinical safety of levo-oxiracetam injection cannot be guaranteed, and the sterility is not achieved, so as to ensure the effectiveness and safety, avoid Good effect of discoloration and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Levoxiracetam injection is prepared according to the following steps:
[0041]
[0042]
[0043] 1. Concentrated formulation: Dissolve edetate calcium sodium, cysteine and thimerosal with 50%-70% of the prescription amount of sterile water for injection, then add levoxiracetam and stir to dissolve;
[0044] 2. Dilute formulation: take the concentrated formulation, add citric acid-sodium citrate solution (citrate ion concentration is 30mmol / L), adjust the pH to 5.4, stir, mix well, add sterilized water for injection to the prescribed amount ;
[0045] 3. Potting: use 0.22μm polycarbonate membrane material microporous filter membrane to filter, fill and seal;
[0046] 4. Sterilization: Send the filled ampoule into a steam sterilizer for sterilization at 121°C for 15 minutes. Sterilization procedure: 10°C / min, rise to 121°C, keep at 121°C for 15 minutes; compressed air blast Wind cooling at 3-5°C / min, cooling to 70-80°C in 8-12 minutes, cooling with cooling water...
Embodiment 2
[0049] Levoxiracetam injection is prepared according to the following steps:
[0050] Element
Dosage
Levoxiracetam
80g
0.2g
cysteine
0.1g
Thimerosal
0.1g
Sterile water for injection
Add to 1000ml
[0051] Preparation process:
[0052] 1. Concentrated formulation: Dissolve edetate calcium sodium, cysteine and thimerosal with 50%-70% of the prescription amount of sterile water for injection, then add levoxiracetam and stir to dissolve;
[0053] 2. Dilute formulation: Take the concentrated formulation, add citric acid-sodium citrate solution (citrate ion concentration is 15mmol / L), adjust the pH to 5.4, stir, mix well, add sterilized water for injection to the prescribed amount ;
[0054] 3. Potting: use 0.22μm polycarbonate membrane material microporous filter membrane to filter, fill and seal;
[0055] 4. Sterilization: Send the filled ampoule into a steam sterilizer for sterilizatio...
Embodiment 3
[0058] Levoxiracetam injection is prepared according to the following steps:
[0059] Element
Dosage
Levoxiracetam
240g
0.4g
cysteine
0.3g
Thimerosal
0.3g
Sterile water for injection
Add to 1000ml
[0060] Preparation process:
[0061] 1. Concentrated formulation: Dissolve edetate calcium sodium, cysteine and thimerosal with 50%-70% of the prescription amount of sterile water for injection, then add levoxiracetam and stir to dissolve;
[0062] 2. Dilute formulation: take concentrated formulation, add citric acid-sodium citrate solution (citrate ion concentration is 50mmol / L), adjust pH to 6.8, stir, mix well, add sterilized water for injection to the prescribed amount ;
[0063] 3. Potting: use 0.22μm polycarbonate membrane material microporous filter membrane to filter, fill and seal;
[0064] 4. Sterilization: Send the filled ampoule into a steam sterilizer for sterilization, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com