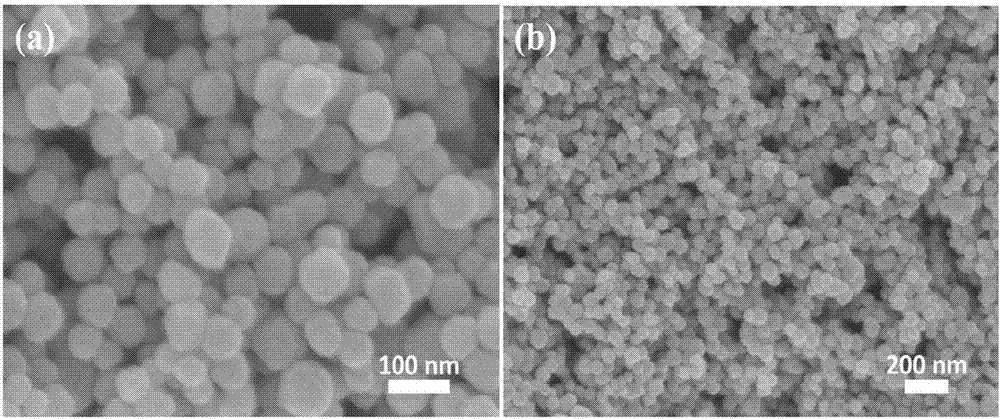
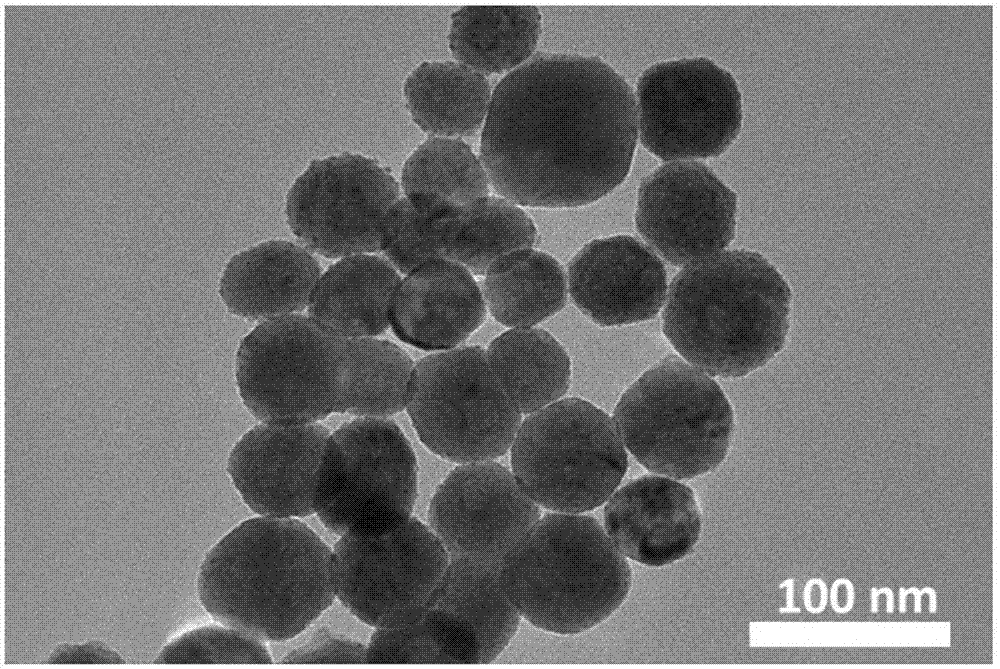
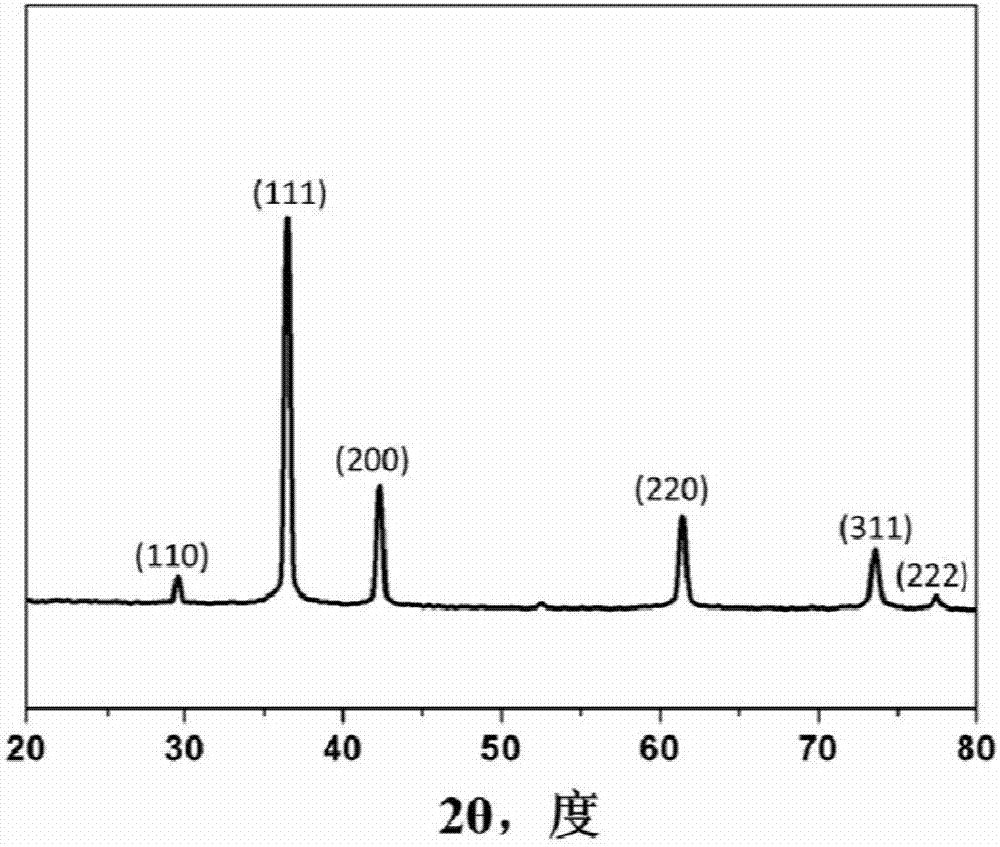
Cuprous oxide nanocrystalline with adjustable morphology and dimension as well as preparation method and application thereof
A technology of cuprous oxide and nanocrystals, which is applied in the field of nanomaterials, can solve the problems of iron impurities, and achieve the effect of green raw materials, mild conditions, and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1) Preparation of copper hydroxide precursor: Add 1.7048g of copper chloride dihydrate into a 150mL single-mouth bottle, and stir and dissolve with 80mL of deionized water. The molar concentration of copper chloride dihydrate is 0.1M. After the solution turns blue and clear, add 20 mL of 2M sodium hydroxide solution dropwise at a rate of 4 mL / min, continue stirring for 30 minutes, and centrifuge at 10,000 r / min to obtain a dark blue suspension. Wherein, the molar ratio of divalent copper ions to hydroxide ions is 1:4.
[0044] 2) Preparation of copper hydroxide dispersion system: Wash the above dark blue suspension once with deionized water, and then redisperse it in 100 mL of deionized water. At this time, the pH value of the dispersion system is 11.8.
[0045] 3) Reduction reaction: under stirring, add 20 mL of 1M ascorbic acid aqueous solution dropwise at a rate of 2 mL / min to the dispersion system, stir at room temperature and continue the reaction for 1 hour, centr...
Embodiment 2
[0048] 1) Preparation of copper hydroxide precursor: Add 0.1600 g of anhydrous copper sulfate into a 25 mL single-mouth bottle, and stir and dissolve with 8 mL of deionized water. The molar concentration of anhydrous copper sulfate is 0.1M. After the solution turns blue and clear, add 2mL of 2M sodium hydroxide solution dropwise at a rate of 0.2mL / min, continue stirring for 60 minutes, and centrifuge at 10000r / min to obtain a dark blue suspension. Wherein, the molar ratio of divalent copper ions to hydroxide ions is 1:4.
[0049] 2) Preparation of copper hydroxide dispersion system: wash the above dark blue suspension once with deionized water, and then redisperse in 10 mL of deionized water to form a dispersion system. Afterwards, 1 mL of 2M NaOH solution was added to adjust the pH of the dispersion to 12.6.
[0050] 3) Reduction reaction: under stirring, add 2.5mL of 0.2M reducing agent ascorbic acid aqueous solution dropwise at a rate of 0.1mL / min to the copper hydroxide d...
Embodiment 3
[0052] 1) Preparation of copper hydroxide precursor: Add 1.1254g of anhydrous copper nitrate into a 100mL single-mouth bottle, and stir and dissolve with 54mL of deionized water. The molar concentration of anhydrous copper nitrate is 0.1M. After the solution turns blue and clear, add 6 mL of 4M sodium hydroxide solution dropwise at a rate of 0.3 mL / min, continue stirring for 30 minutes, and centrifuge at 10,000 r / min to obtain a dark blue suspension. Wherein, the molar ratio of divalent copper ions to hydroxide ions is 1:4.
[0053] 2) Preparation of copper hydroxide dispersion system: wash the above dark blue suspension with deionized water once, and then redisperse in 60mL deionized water to obtain a dispersion system; under stirring, add 6mL 0.1M hydrochloric acid to adjust the pH value of the dispersion system to 10.6.
[0054] 3) Reduction reaction: under stirring, add 12mL of 0.25M ascorbic acid aqueous solution dropwise to the dispersion system at a rate of 1mL / min, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com