Coumarin derivative, preparation and application of coumarin derivative, and bisulfite detection kit
A bisulfite and coumarin-based technology, applied in chemical instruments and methods, measuring devices, color/spectral characteristics measurement, etc., can solve the problem that the sensitivity is not enough to meet the detection requirements of samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A kind of coumarin derivative, its chemical name is 2-((1E,3Z)-4-chloro-4-(6-diethylamino)-2-oxo-2H-chroman -3) butane-1,3-diene-1)-3-methylbenzo[d]thiazolium-ium iodide (2-((1E,3Z)-4-chloro-4-(6-( diethylamino)-2-oxo-2H-chromen-3-yl)buta-1,3-dien-1-yl)-3-methylbenzo[d]thiazol-3-ium), its structural formula is as follows:
[0055] .
[0056] The preparation method of coumarin derivatives shown in formula (I) comprises the following steps:
[0057] (1) Dissolve 5mmol 4-(diethylamino)salicylaldehyde and 5mmol ethyl acetoacetate in 20mL ethanol, add 0.1mL piperidine, stir and reflux the resulting mixture for 8h, stop the reaction, cool to room temperature, and filter to obtain the compound 1 (3-acetyl-7-(diethylamino)-2H-chroman-2-one, 3-acetyl-7-(diethylamino)-2H-chromen-2-one);
[0058] (2) Stir 2 mL of DMF and 2 mL of phosphorus oxychloride at 0°C for 30 min, and add dropwise to compound 1 (3-acetyl-7-(diethylamino)-2H-benzobis) dissolved in 20 mL of DMF Hydropyra...
Embodiment 2
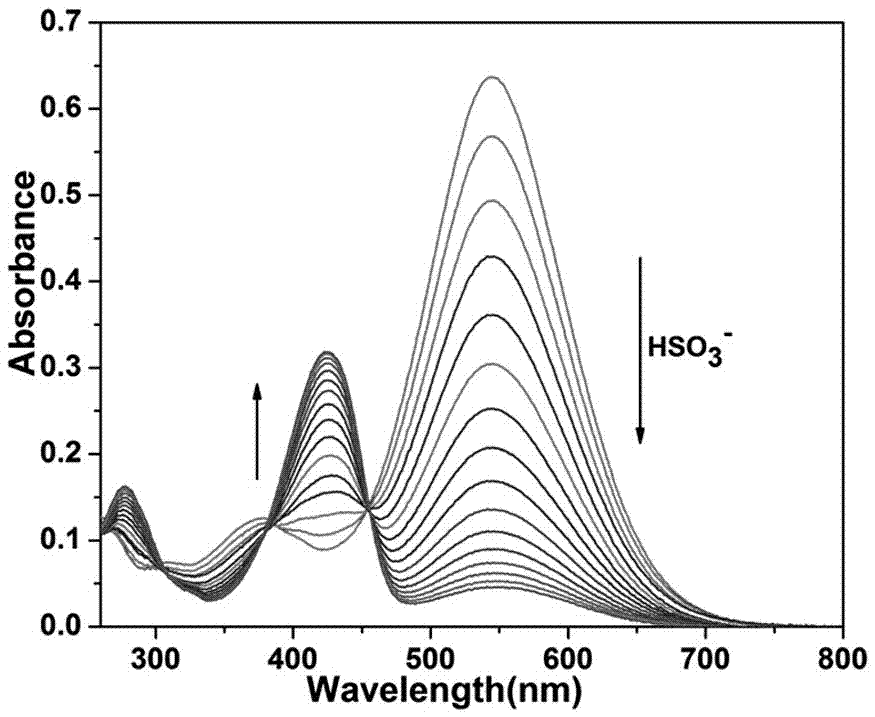
[0067] Prepare HEPES buffer solution with pH = 7.4 and a concentration of 10mM for use; prepare a DMF solution of coumarin derivatives shown in formula (I) with a concentration of 2mM for use; prepare 1000µL of Add DMF solution, 1000µL HEPES buffer solution and 10µL DMF solution of coumarin derivatives shown in formula (I) to a clean UV cuvette, and detect it on a UV-Vis spectrophotometer while adding samples. With the addition of hydrogen sulfate solution, the absorption peak at 545nm gradually decreases, and the absorption peak at 424nm gradually increases, see figure 1 .
Embodiment 3
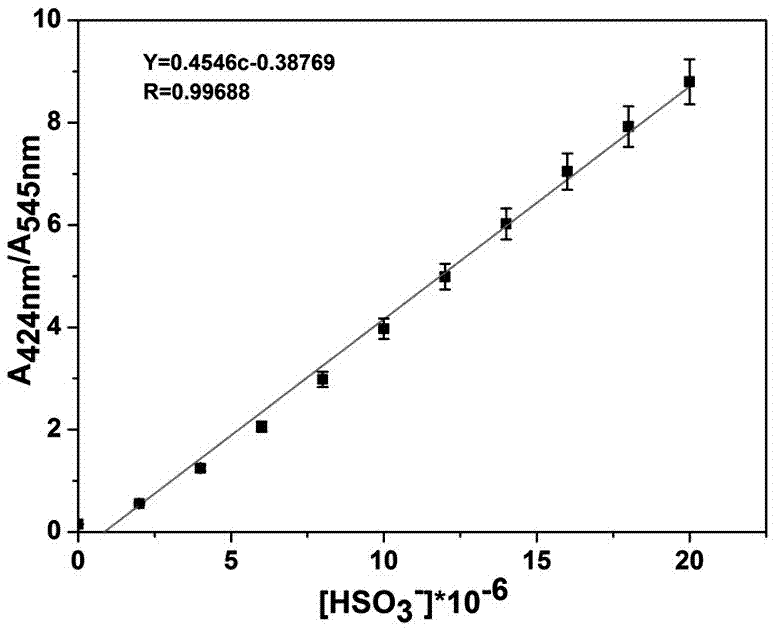
[0069] Configure pH = 7.4, HEPES buffer solution with a concentration of 10mM, for standby; configure a DMF solution of coumarin derivatives shown in formula (I) with a concentration of 2mM, for standby; mix 1000µL DMF solution, 1000µL HEPES buffer solution and 10µL formula ( I) Add the DMF solution of the coumarin derivatives shown in a clean UV cuvette, and then gradually add standard aqueous bisulfite (HSO 3- Concentration is 2mM), when the accumulatively added volumes of standard bisulfite aqueous solution are 0µL, 2µL, 4µL, 6µL, 8µL, 10µL, 12µL, 14µL, 16µL, 18µL, 20µL, the UV absorption curves were detected respectively, and the results Such as figure 1 As shown, the absorption peaks corresponding to 545nm measured on a UV-visible spectrophotometer are 0.646, 0.379, 0.225, 0.153, 0.111, 0.087, 0.07, 0.059, 0.051, 0.046, 0.041; 424nm The corresponding absorption peaks are 0.099, 0.210, 0.281, 0.315, 0.333, 0.346, 0.353, 0.359, 0.362, 0.365, 0.366;
[0070] Then take the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com