Fluorescent probe based on rhodamine-indole derivative as well as preparation method and application of fluorescent probe
A technology of indole derivatives and fluorescent probes, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effects of strong selectivity, easy availability of raw materials, and wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of Rhodamine-Indole Derivatives
[0025]
[0026] Indole-3-carboxylic acid (161mg, 1mmol), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (191mg, 1mmol) and 4-dimethylaminopyridine (122mg, 1mmol) was dissolved in dichloromethane (20ml), then rhodamine B ethylenediamine (456mg, 1mmol) was added, refluxed at normal pressure for 12h, concentrated in vacuo, purified by silica gel column chromatography [CH 2 Cl 2 / CH 3 OH=100:1 (v / v)] to obtain a yellow solid which is the target product, and the yield of the target product is 69%.
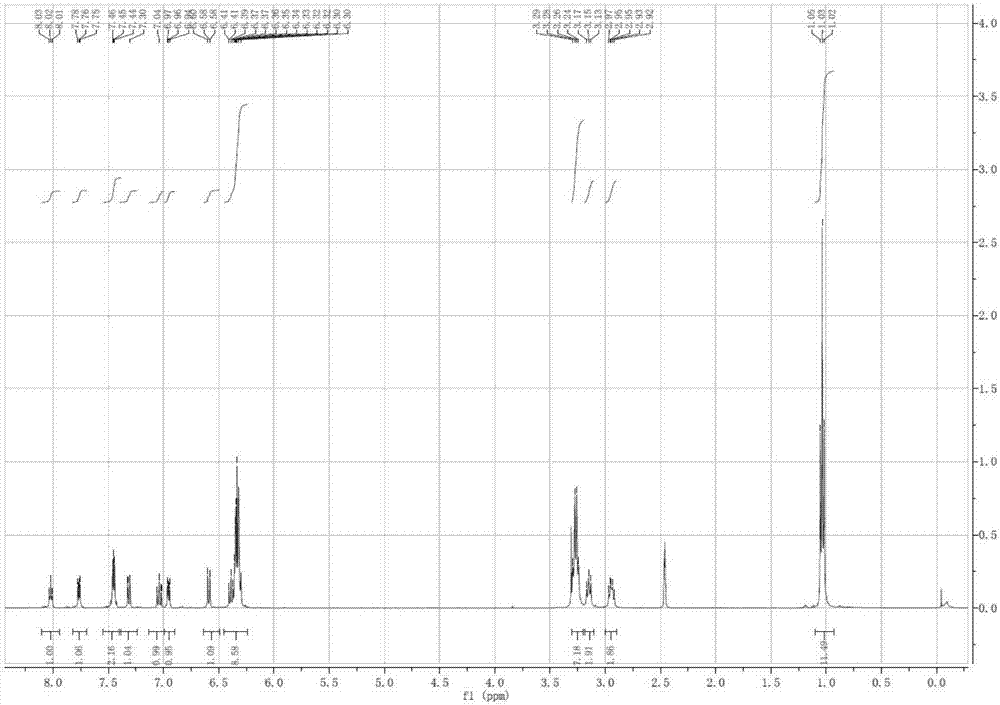
[0027] Rhodamine-indole derivative NMR analysis results are as follows:
[0028] Proton NMR spectrum: 1 H NMR (400MHz, d 6 -DMSO),δ(ppm):8.01-8.03(t,1H,NH),7.75-7.76(m,1H,Aryl-H),7.44-7.46(m,2H,Aryl-H),7.30-7.32( m,1H, Aryl-H),7.02-7.06(t,1H,Aryl-H),6.94-6.97(m,1H,Aryl-H),6.58-6.60(m,1H,Aryl-H),6.39- 6.41(m,1H,Aryl-H),6.30-6.37(m,7H,Aryl-H),3.24-3.29(d,8H,4CH 2 ),3.13-3.17(m,2H,CH 2 ),2.92-2.97(m,2H,CH 2 ),1.02-...
Embodiment 2
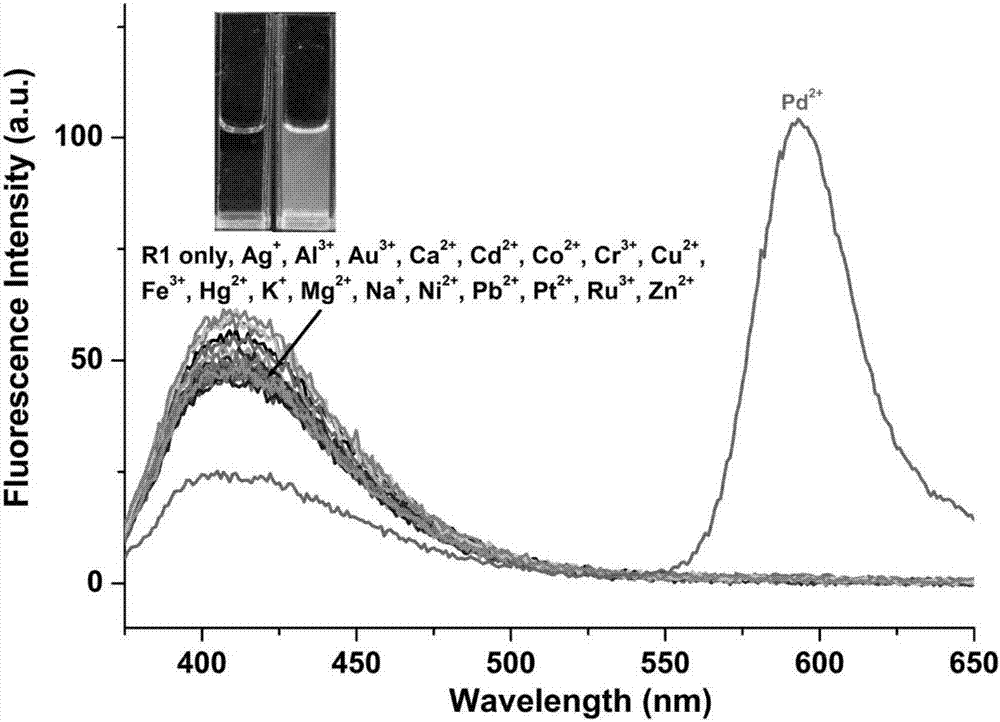
[0031] Determination of Optical Properties of Rhodamine-Indole Derivatives on Divalent Palladium Ions
[0032] The rhodamine-indole derivatives prepared in Example 1 above were used as probes in acetonitrile / water (volume ratio 9:1) medium to prepare a molar concentration of 5×10 -6 mol / L solution, respectively, at a molar concentration of 5×10 -6 mol / L Ag + , Al 3+ , Au 3+ , Ca 2+ , Cd 2+ ,Co 2+ , Cr 3+ , Cu 2+ , Fe 3+ , Hg 2+ , K + , Mg 2+ ,Mn 2+ , Na + , Ni 2+ , Pb 2+ , Pd 2+ , Pt 2+ , Zn 2+Add an equal amount of the above-mentioned probe solution in the solution of metal ions, adopt the fluorescence spectrometer to carry out the fluorescence spectrum analysis to the rhodamine-indole derivative that embodiment 1 makes, the fluorescence spectrum figure of gained is shown in image 3 . The rhodamine-indole derivatives prepared in Example 1 of the present invention interact with divalent palladium ions, and the emission peak at 590nm increases significantl...
Embodiment 3
[0034] The concentration is 5×10 -6 The rhodamine-indole derivatives of mol / L are added concentration respectively in the acetonitrile / water (volume ratio 9:1) solution of fluorescence probe as 0mol / L, 5×10 -7 mol / L, 1×10 -6 mol / L, 1.5×10 -6 mol / L, 2×10 -6 mol / L, 2.5×10 -6 mol / L, 3×10 -6 mol / L, 3.5×10 -6 mol / L, 4×10 -6 mol / L, 4.5×10 -6 mol / L, 5×10 - 6 mol / L, 7.5×10 -6 mol / L, 1×10 -5 mol / L, 1.25×10 -5 mol / L, 1.5×10 -5 The divalent palladium ion of mol / L adopts fluorescence spectrometer to carry out fluorescence spectrum analysis to it respectively, record the fluorescence intensity value at 410 and 590nm place, the fluorescence spectrum figure of gained is shown in Figure 4 . by attaching Figure 4 It can be seen that as the palladium ion concentration increases, the fluorescence intensity of the rhodamine-indole derivative fluorescent probe at 410nm gradually weakens, and the fluorescence intensity at 590nm gradually increases, and the absorption peak intensit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com