Fat SVF cell clinical high-efficiency preparation and cryopreservation method, and applications thereof
A fat and cell technology, applied in the field of the preparation of fat SVF cells, can solve the problems of low cell viability, low SVF purity, and large amount of collagenase used, so as to improve digestion ability, strong cell proliferation activity, and prevent excessive cell proliferation. effects of digestion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] (1) Sample screening
[0071] Medical history collection and testing of fat donors: including basic information of the donor, past medical history, family history, etc.
[0072] Past history and family history should collect detailed information about genetic diseases (single gene and polygenic diseases, including cardiovascular diseases and tumors, etc.).
[0073] Inspection and screening prove that the infection of specific unmanned viruses (including HIV, HBV, HCV, HTLV, EBV, CMV, etc.) is free of Treponema pallidum infection. If necessary, the donor's ABO blood type, HLA-I and II types need to be collected Data for retrospective inquiry.
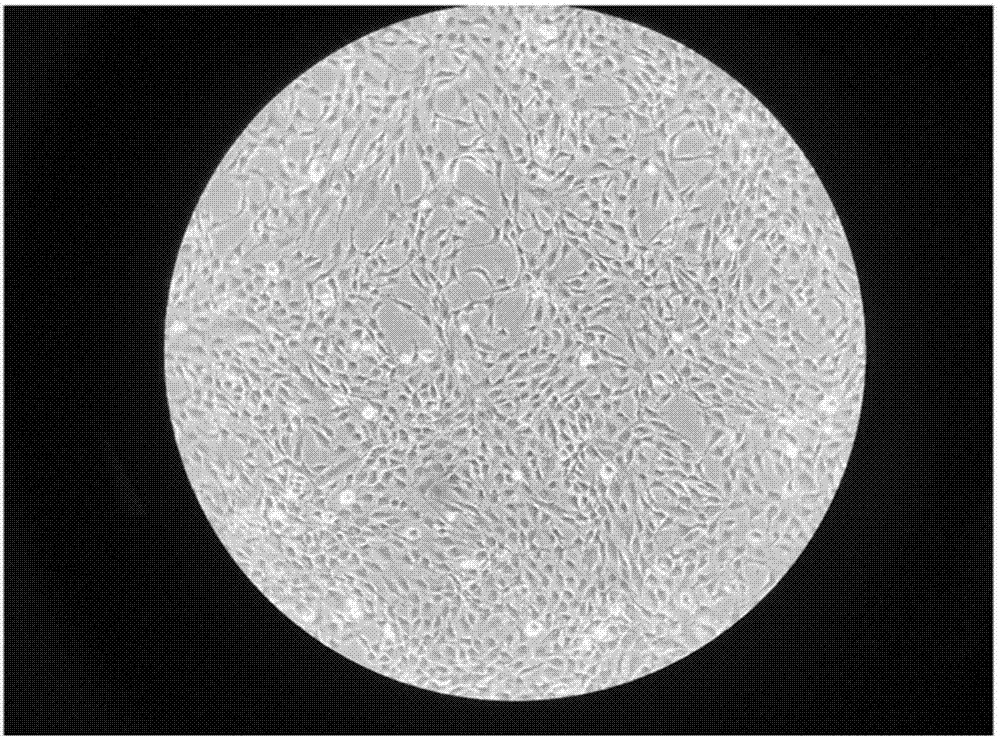
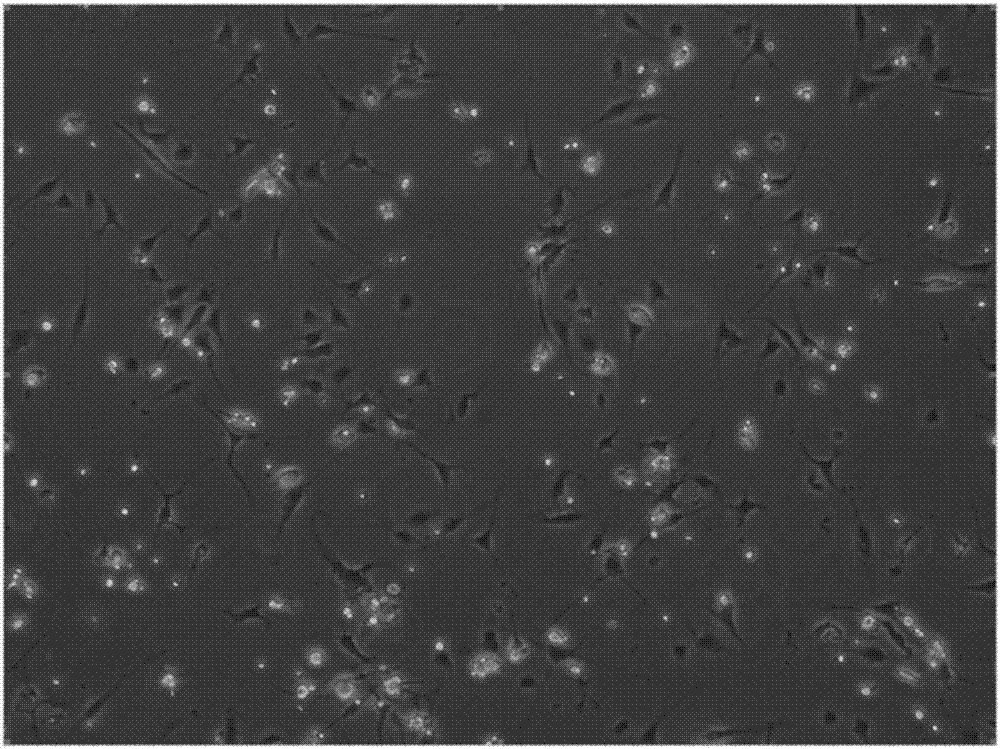
[0074] (2) A method for clinically efficient preparation of fat SVF cells
[0075] Preparation of experimental materials:
[0076] The preparation of the preservation solution: It is composed of 0.9% normal saline + 100ug / mL penicillin + 100ug / mL streptomycin.
[0077] Preparation of washing solution: 500ml of normal saline, add 5ml of 20% h...
Embodiment 2
[0110] (1) Cryopreservation method of human adipose SVF cells
[0111] A method for freezing human adipose SVF cells, the freezing method includes:
[0112] Step S1': Use clinical grade DMSO (Dimethyl Sulfoxide, Article No. WAK-DMSO-10, Manufacturer Cryosure) and Serum Replacement (KnockOut™ Serum Replacement, KSR, Article No. 10828-028, Manufacturer Gibco) in accordance with 1:9 Mix by volume to form a freezing solution;
[0113] Step S2': slowly add the above cryopreservation solution to the centrifuge tube containing human fat SVF cells at a ratio of 1:1, pipette to mix the cells, mix well, and add the mixed cell suspension to the 2mL cryopreservation tube. Add 1.5mL to each tube, tighten the lid after adding, seal with the sealing film, mark the sample number, name, and freeze storage time, and put the marked cryotube into the freezer box;
[0114] Step S3': Place the sample in the cryopreservation box in the program cooling device to perform program cooling. The program cooling ...
Embodiment 3
[0121] A pharmaceutical preparation comprising: fat SVF cells, normal saline and albumin; wherein the mass ratio of normal saline to albumin is 98:2.
[0122] The human adipose SVF cells are prepared by the clinical-grade high-efficiency preparation method of human adipose SVF cells described in Example 1. The concentration of fat SVF cells is 0.5*10 7 / mL ~1.5*10 8 / mL.
[0123] The drug is used as a preparation for the treatment of osteoarthritis. Its dosage is in accordance with the clinical treatment dosage, and the dosage is 1*10 in a single time. 8 / 2mL ~2*10 8 / 2mL.
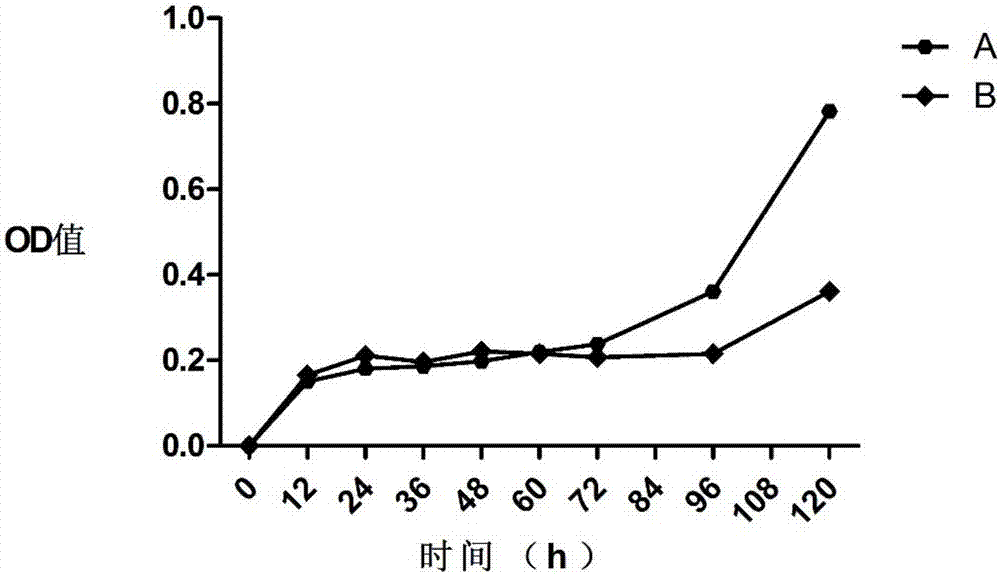
[0124] In summary, the clinical-grade efficient preparation and cryopreservation method of human adipose SVF cells of the present invention and its use. The washing solution and digestion solution used in the preparation method make the prepared adipose SVF cells have high cell viability and purity. Improved.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com