A reflectin extracting method
An extraction method and protein technology, which is applied in the field of extracting reflectin protein, can solve the problems of low solubility and inability to obtain reflectin protein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Expression of Reflexin
[0026] The full-length SoRef2 gene (from cuttlefish Sepia officinalis, GenBank ID: HE687200.1) was synthesized by Beijing Biomed Gene Technology Co., Ltd., and cloned into the expression vector pCOLD1 (Takara), with a His fusion tag at its N-terminal, The promoter is cold-inducible promoter cspA, the operon is lacZ, and the expression system is Escherichia coli BL21 (DE3) strain. When the OD600 of the bacteria reached 0.6-0.8, they were induced with IPTG at a final concentration of 20 μM at a temperature of 288K for 20 hours, and then the bacteria were harvested.
Embodiment 2
[0027] Embodiment 2: Purification of reflectin protein
[0028] The harvested bacteria were resuspended in buffer T (20mM Tris, 150mM NaCl, pH 8.0), and placed on ice for ultrasonic lysis. The parameters of ultrasonic lysis were: 200-220w, working time 10s, gap time 5s, cycle 100 times .
[0029] After the lysate was centrifuged at 15000rpm for 30min, the precipitate was retained, and the precipitate was resuspended in buffer R (20mM Tris, 150mM NaCl, 0.5% Triton X-100, pH 8.0), incubated in a rotary shaker at room temperature for 30min, and centrifuged again , to collect the precipitate. The resulting precipitate was washed three times with ultrapure water, then resuspended in buffer S (20 mM Tris, 150 mM NaCl, 0.05% SDS, pH 8.0), and incubated on a rotary shaker at room temperature for 30 min to dissolve the luciferin.
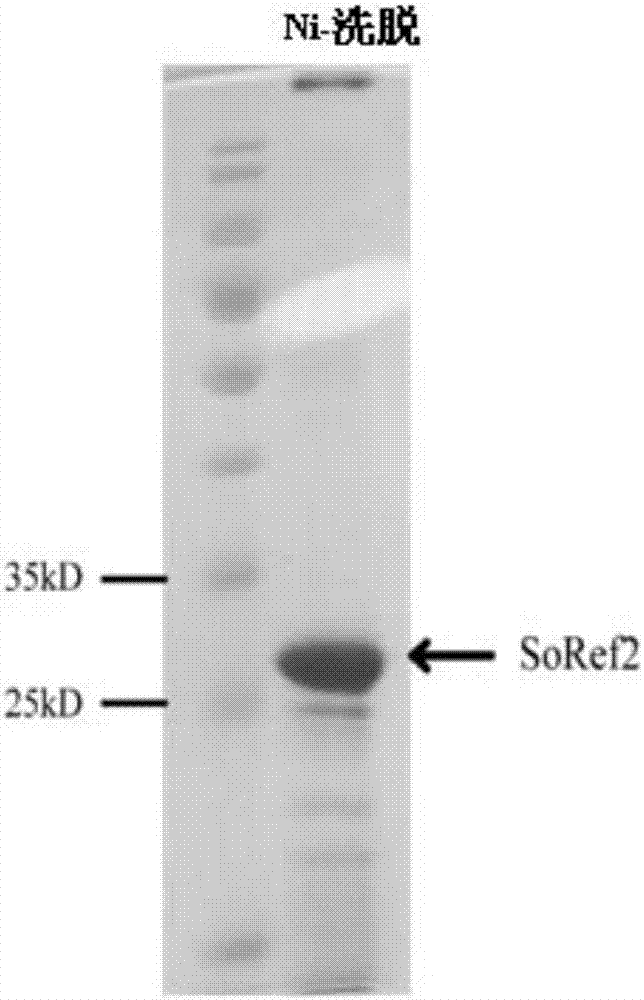
[0030] After centrifugation at 15,000 rpm for 30 min, the supernatant containing the reflectin protein was taken, and imidazole was added thereto to a fin...
Embodiment 3
[0032] Example 3: Further purification and verification of reflectin protein
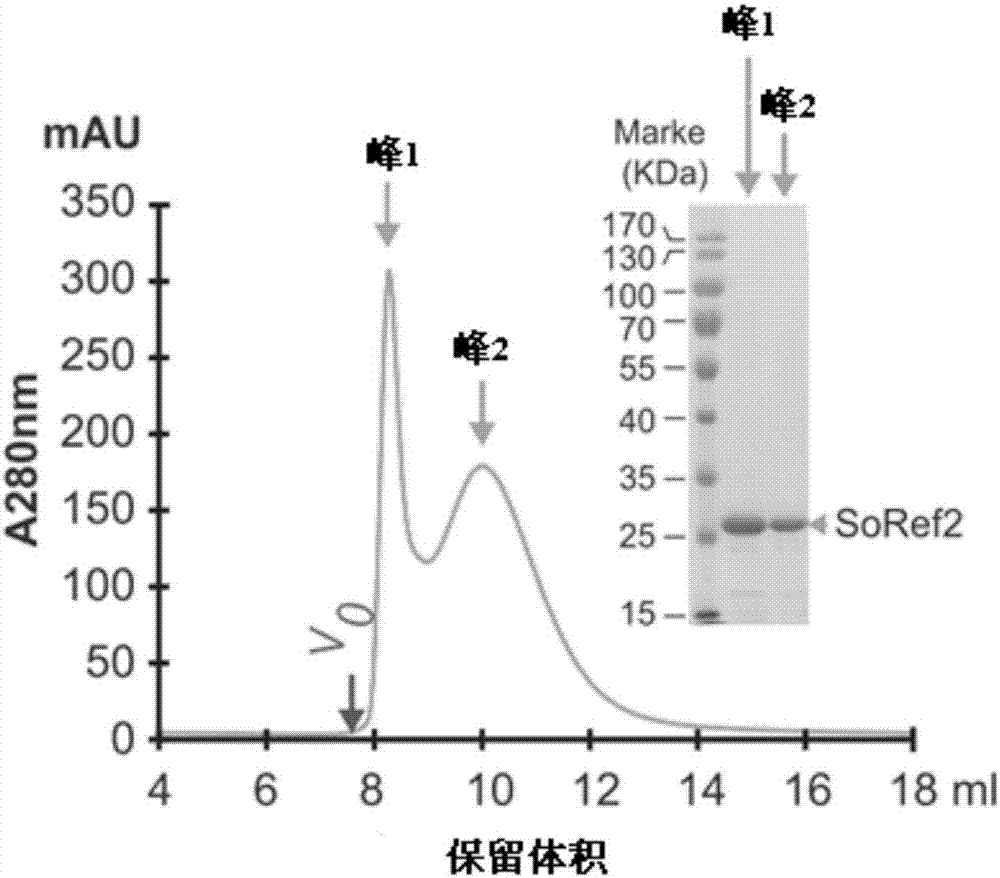
[0033] In order to further obtain the protein with uniform particles, the particle distribution analysis and separation of the reflectin protein were carried out using a gel filtration chromatography column Superdex 200 10 / 300 GL (GE Healthcare, Sweden), and the mobile phase buffer used was 20mM Tris, 0.1 % SDS, pH 8.0.
[0034] Such as figure 2 As shown, the obtained reflectin protein presents two peaks, which represent two distributions of protein particle size, and the first peak is relatively narrow and symmetrical, indicating that the protein particles distributed here are relatively more uniform. Gel filtration chromatography column analysis is used to separate particles of different sizes, and at the same time achieve the effect of further purification and remove impurity proteins. Comparedfigure 1 and figure 2 According to the electrophoresis gel map, after this step, figure 2 The pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com