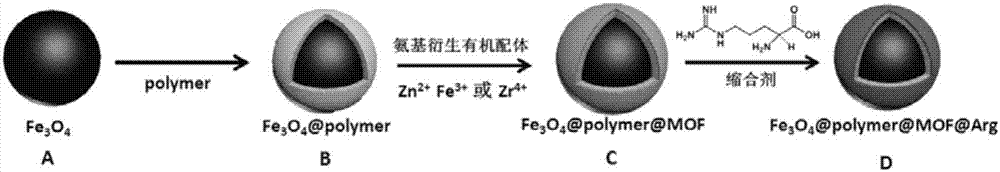
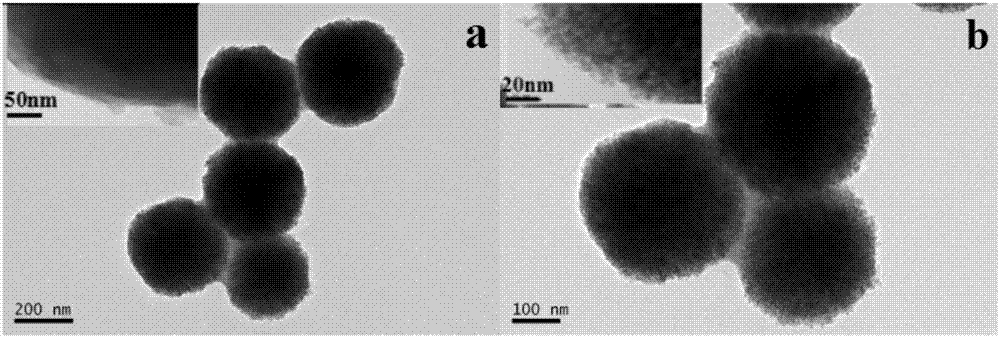
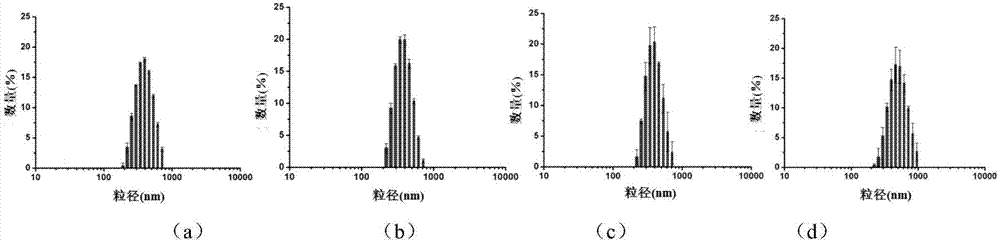
Magnetic metal-organic framework nanosphere with multiple affinity sites as well as preparation method and application thereof
An organic framework and magnetic metal technology, applied in chemical instruments and methods, alkali metal compounds, alkali metal oxides/hydroxides, etc., can solve problems such as limited applications, low enrichment efficiency of phosphorylated polypeptides, and cumbersome operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1- Embodiment 8
[0046] Embodiment 1-embodiment 8 prepares Fe 3 o 4 / Polymer nanoparticles
[0047] Take the raw material according to table 1, and prepare Fe according to the following method in conjunction with the process parameters given in table 1 3 o 4 / Polymer nanoparticles: under stirring at room temperature, according to polyvinylpyrrolidone and Fe 3 o 4 The mass ratio of magnetic balls (5~10): 1, adding Fe to the high molecular polymer solution containing polyvinylpyrrolidone 3 o 4 Magnetic balls, continue stirring for at least 6 hours to obtain the first mixed solution, magnetically separate the first mixed solution and collect the separated solid product, and then wash the solid product to remove the uncoated Fe 3 o 4 The polymer on the surface of the magnetic ball can obtain the Fe covered by the middle layer of polymer polymer 3 o 4 Magnetic ball, namely Fe 3 o 4 / Polymer nanoparticles; the mass concentration of the polymer solution is 8mg / ml~80mg / ml, which is formed b...
Embodiment 9- Embodiment 12
[0054] Embodiment 9-embodiment 12 prepares Fe 3 o 4 / Polymer / MOFs
[0055] Take the raw material according to table 3, and prepare Fe according to the following method in conjunction with the process parameters given in table 3 3 o 4 / Polymer / MOFs: Fe 3 o 4 / Polymer nanoparticles are uniformly dispersed in DMF to obtain Fe 3 o 4 / Polymer nano particle suspension, under the condition of stirring, Fe 3 o 4 / Polymer nanoparticle suspension is added to the DMF solvent or the mixed solution obtained by mixing DMF and deionized water uniformly to obtain the second mixed solution, and the soluble inorganic salt containing metal ions is added to the second mixed solution, and stirred for 3 minutes to 10 minutes Then add amino-derived organic ligands, continue to react at 100-150°C under stirring conditions for at least 1 hour to obtain the first reaction liquid, perform magnetic separation on the first reaction liquid and collect the separated solid products, and then use the ...
Embodiment 13
[0058] Embodiment 13 prepares Fe 3 o 4 / Polymer / MOFs
[0059] This embodiment prepares Fe 3 o 4 The steps of / Polymer / MOFs are as follows: the Fe prepared by 400mg embodiment 8 3 o 4 / Polymer nanoparticles are uniformly dispersed in 1ml DMF to obtain Fe 3 o 4 / Polymer nano particle suspension, mix 13mlDMF, 2ml deionized water to obtain a mixed solvent, add acetic acid to the mixed solvent to adjust the pH value of the mixed solvent to 4.6, under the stirring condition of 800rmp, Fe 3 o 4 / Polymer nano particle suspension joins in the mixed solvent after adjusting the pH value and obtains the second mixed solution, adds 2.24mmol Zn(NO 3 ) 2 ·6H 2 O, after stirring at 800rmp for 5min, add 1.12mmol NH 2 -BDC, continue to react at 100°C for 1 hour under the stirring condition of rotating speed of 800rmp to obtain the first reaction solution, carry out magnetic separation on the first reaction solution and collect the separated solid product, and then use DMF, ethanol, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com