Preparation method of sulfur-containing 3-arylmethylene isoindolinone derivative
A technology for arylmethylene isoindolinone and derivatives, which is applied in the field of preparing sulfur-containing 3-arylmethylene isoindolinone derivatives, can solve problems such as catalyst poisoning, and achieves reduction of emissions and easy product availability. Obtain and protect the health of operators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
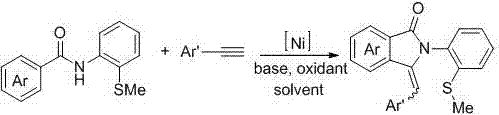
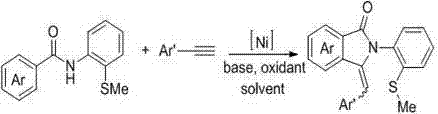
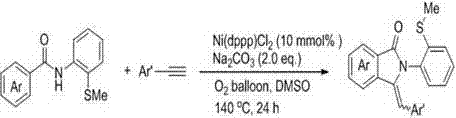
[0020] The present invention provides a kind of preparation method of sulfur-containing 3-aryl methylene isoindolinone derivatives, comprising the following steps: N -(2-Methylthio-phenyl)aryl formamide and aryl terminal alkynes as substrates; by adding 10 mol% Ni(dppp)Cl 2 Metal catalyst, 200 mol% Na 2 CO 3 Alkaline, O 2 Oxidant, DMSO is the reaction solvent, at a temperature of 100 o C~140 o Under the condition of C, react for 12~24 h; the chemical reaction formula is as follows:
[0021]
[0022] The Ar is phenyl, 4-methylphenyl, 4-methoxyphenyl, 4-fluorophenyl, 4-chlorophenyl, 3-chlorophenyl, 4-bromophenyl, 4-nitro One of phenyl, 4-biphenyl, 2-thienyl; Ar' is 4-methylphenyl, 4-methoxyphenyl, 4-fluorophenyl, 4-chlorophenyl, 4- One of bromophenyl, 4-nitrophenyl, 4-trifluoromethylphenyl;
[0023] After the reaction was completed, it was cooled, diluted with ethyl acetate, transferred to a separatory funnel and washed three times with saturated brine, the aqueous l...
specific Embodiment 1
[0024] Specific embodiment one: 48.7 milligrams (0.2 mmol) N -(2-Methylthio-phenyl)benzamide, 61.3 mg (0.6 mmol) phenylacetylene, 10.8 mg (0.02 mmol) Ni(dppp)Cl 2 , 42.4 mg (0.4 mmol) of anhydrous sodium carbonate was added to 2 mL of dimethyl sulfoxide solvent. Three pumps and three links in an oxygen atmosphere, after plugging in an oxygen balloon, at 140 o C oil bath was reacted for 24 hours. After the reaction was cooled to room temperature, it was diluted with 10 mL of ethyl acetate, transferred to a separatory funnel and washed three times with saturated brine, the aqueous layer was extracted once with ethyl acetate, the organic phases were combined, dried with anhydrous sodium sulfate, filtered, and the filtrate Rotary evaporation, removal of solvent, silica gel column chromatography for the residue, sequential washing with a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 10:1 and 5:1, the effluent was collected according to the actual grad...
specific Embodiment 2
[0025] Specific example two: 51.5 mg (0.2 mmol) N-(2-methylthio-phenyl) p-toluamide, 61.3 mg (0.6 mmol) phenylacetylene, 10.8 mg (0.02 mmol) Ni(dppp) Cl 2 , 42.4 mg (0.4 mmol) of anhydrous sodium carbonate was added to 2 mL of dimethyl sulfoxide solvent. Three pumps and three links in an oxygen atmosphere, after plugging in an oxygen balloon, at 140 o C oil bath was reacted for 24 hours. After the reaction was cooled to room temperature, it was diluted with 10 mL of ethyl acetate, transferred to a separatory funnel and washed three times with saturated brine, the aqueous layer was extracted once with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, and filtered. The filtrate was rotary evaporated, the solvent was removed, and the residue was subjected to silica gel column chromatography, followed by washing with a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 10:1 and 5:1, and the effluent was collected accord...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com