Preparation method of photoelectrode loaded with zinc-nickel-cobalt basic carbonate
A carbonate and photoelectrode technology, which is applied in the direction of electrodes, electrolytic components, and electrolytic processes, can solve the problems of high cost, lack of stability, and low hydrogen conversion efficiency, and achieve sufficient raw materials, low prices, and improved photolysis of water. efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Clean the FTO conductive glass ultrasonically for 20 minutes in the order of acetone, alcohol and deionized water. Add 1 mL of tetrabutyl titanate and 0.269 g of anhydrous citric acid into a mixed solvent of 30 mL of deionized water and 30 mL of hydrochloric acid (36-38% by mass) and continue stirring until uniformly mixed. Use a pipette gun to measure 10mL of the prepared solution and transfer it to a 20mL stainless steel autoclave lined with polytetrafluoroethylene, place the cleaned FTO conductive glass in the autoclave liner with the conductive surface facing down, and then place it in the autoclave liner. After the autoclave is sealed, place it in an oven, raise the temperature to 150°C in 20 minutes, and react at this temperature for 6 hours. After the reaction is completed and naturally cool to room temperature, take the sample out of the autoclave and clean it with deionized water and alcohol , and then dry the cleaned samples in air at 60°C for 2 hours. In...
Embodiment 2
[0042] Prepare TiO according to the method of embodiment 1 step (1)-(2) 2 / ZNC-CH composite electrode, the difference is that the water bath reaction time in step (2) is 10 minutes.
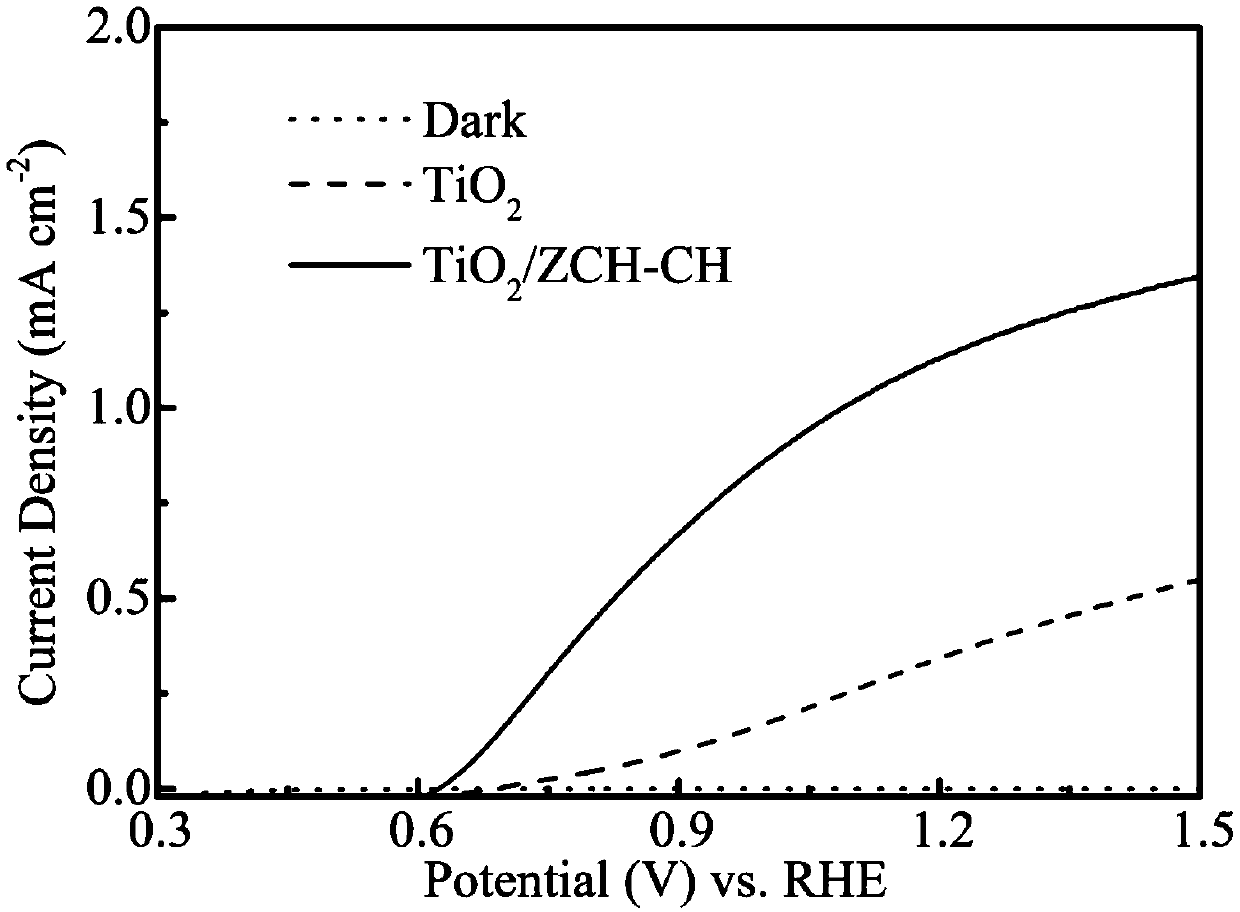
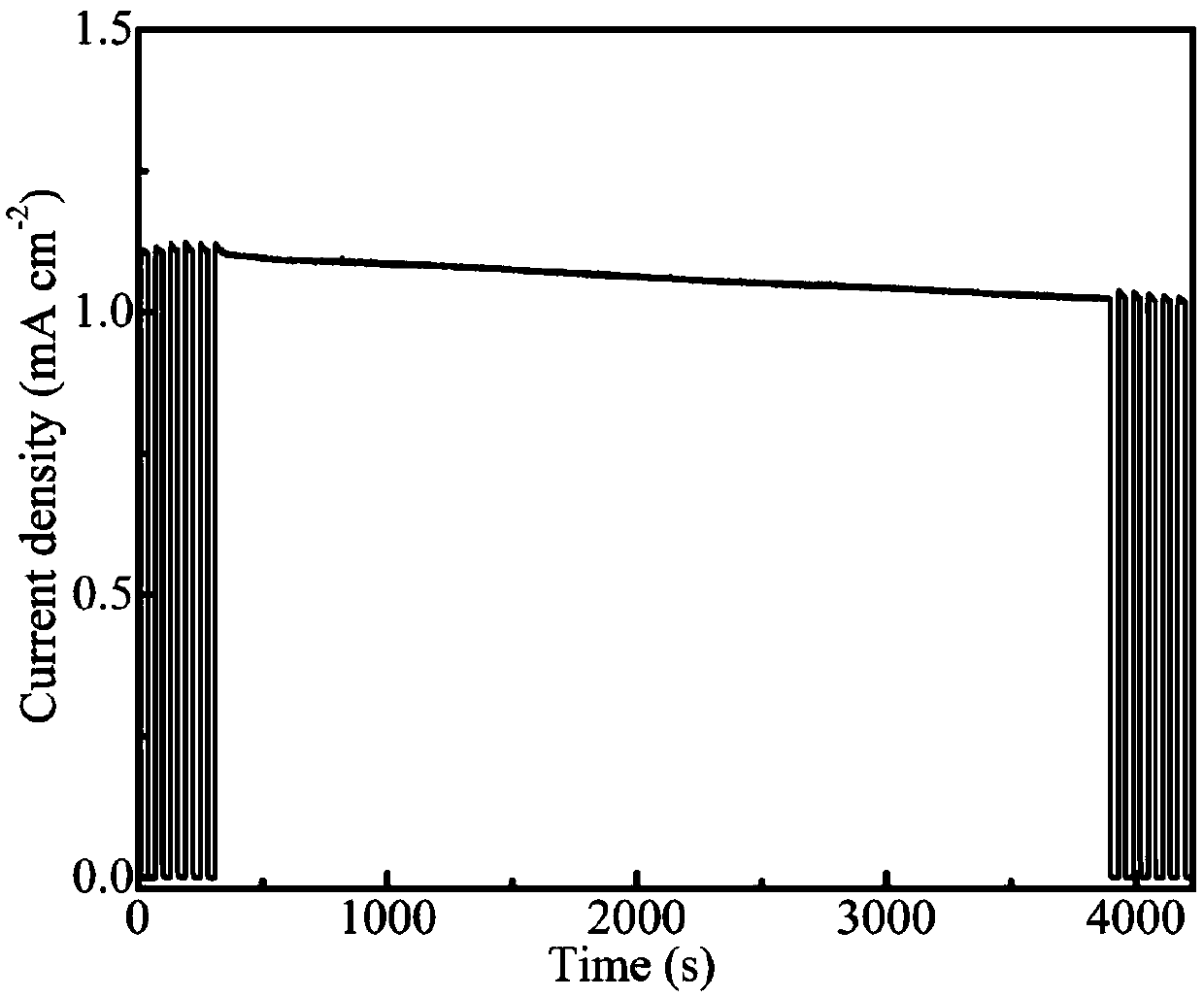
[0043] The above-prepared photoelectrodes were assembled into photoelectrochemical cells, and then water was split under different voltages. Under the voltage of 1.23V, the photocurrent of the photoelectrode prepared by the method of the present invention can reach 0.898mA / cm 2 ; while TiO 2 The photocurrent of the nanorod array is only 0.336mA / cm 2 , the composite electrode of the present invention is TiO 2 2.7 times that of the electrode photocurrent.
Embodiment 3
[0045] Prepare TiO according to the method of embodiment 1 step (1)-(2) 2 / ZNC-CH composite electrode, the difference is that the water bath reaction time in step (2) is 30 minutes.
[0046] The above-prepared photoelectrodes were assembled into photoelectrochemical cells, and then water was split under different voltages. Under the voltage of 1.23V, the photocurrent of the photoelectrode prepared by the method of the present invention can reach 0.538mA / cm 2 ; while TiO 2 The photocurrent of the nanorod array is only 0.336mA / cm 2 , the composite electrode of the present invention is TiO 2 1.6 times the electrode photocurrent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com