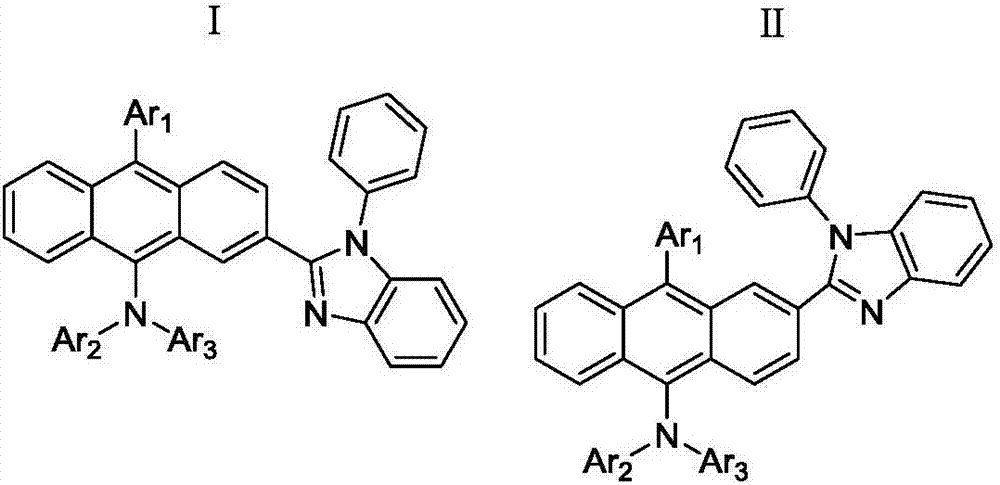
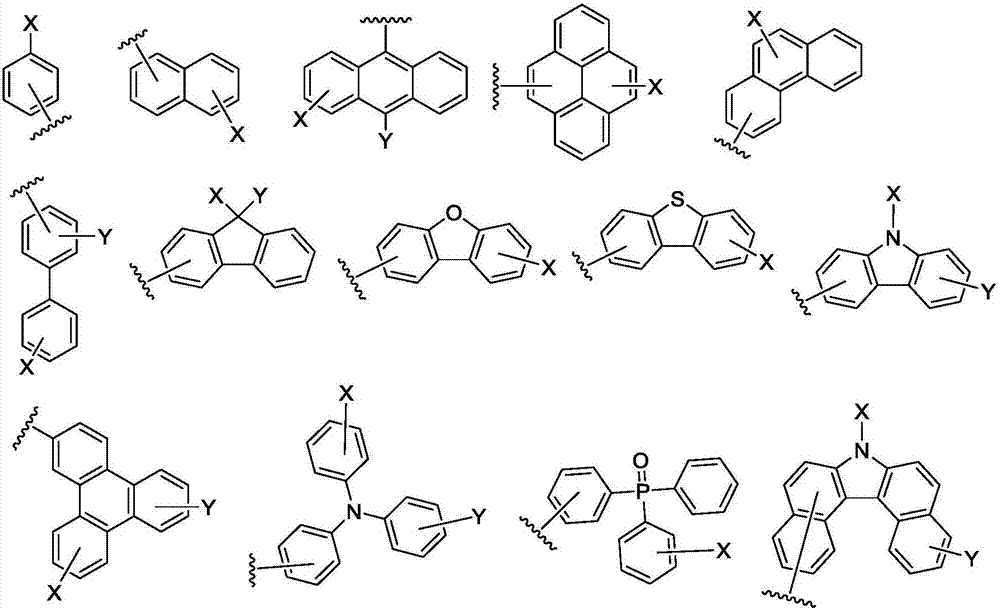
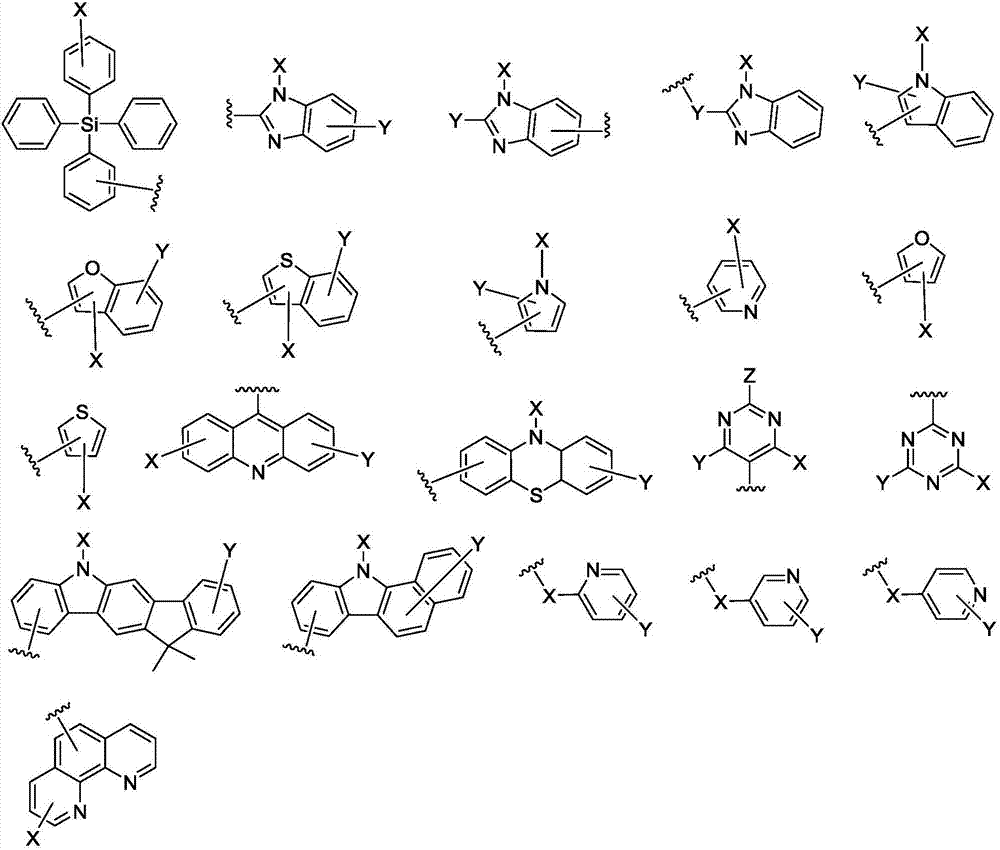
Anthracene compound, synthesis method thereof and organic light emitting device
A technology of organic light-emitting devices and compounds, applied in the field of organic photoelectric materials, can solve the problems of low electron and hole recombination efficiency, low luminous efficiency of light-emitting devices, and inability to effectively balance carriers, etc., to achieve enhanced charge transfer directionality, Difficult to crystallize and reduce planarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] [Example 1] Synthesis of Compound 2
[0054]
[0055] Step1: Under the protection of nitrogen, add 2-bromoanthracene (2.16g, 8.4mmol), 1-phenyl-1H-benzimidazole boronic acid (2.19g, 9.2mmol), tetrakistriphenylphosphine palladium (0.09g , 0.08mmol), potassium carbonate (3.48g, 25.2mmol), toluene 60mL, ethanol 20mL and distilled water 20mL, stirred at 120°C for 3h. After the reaction was finished, stop the reaction with distilled water, extract with ethyl acetate, and use MgSO 4 After drying, the solvent was distilled off under reduced pressure, and then purified by silica gel column chromatography to obtain Intermediate 2-2 (2.04 g, 67%).
[0056] Step2: Under nitrogen and light-free conditions, add 2-2 (3.7g, 10.0mmol), DMF solution 60mL, glacial acetic acid 60mL, chloroform 50mL, add NBS (2.22g, 12.5mmol) into the reactor, stir at room temperature for 2h, The crude product was precipitated, filtered and washed with methanol to obtain intermediate 2-3 (3.46 g, 77%)...
Embodiment 2
[0060] [Example 2] Synthesis of compound 8
[0061]
[0062] Step1: Add 9,9-dimethyl-9H-fluoren-3-amine (1.67g, 8mmol) in toluene solution, 4-bromopyridine (1.05g, 6.67mmol), Pd 2 (dba) 3 (0.17g, 0.2mmol), P(t-Bu)3 (0.14, 0.67mmol), NaOt-Bu (2.24g, 20mmol), 100mL of toluene solution, reacted at 100°C for 24h, after the reaction was completed, diethyl ether and water The organic phase was extracted, and the organic layer was washed with MgSO 4 Drying, concentration of organic matter, column chromatography and recrystallization gave intermediate 8-1 (1.43 g, 75%).
[0063] Step2: Obtain intermediate 8-2 according to the synthesis method of intermediate 2-2.
[0064] Step3: Obtain intermediate 8-3 according to the synthesis method of intermediate 2-3.
[0065] Step4: Obtain intermediate 8-4 according to the synthesis method of intermediate 2-4.
[0066] Step5: Obtain intermediate 8-5 according to the synthesis method of intermediate 2-5.
[0067] Step6: Intermediate 8 (3...
Embodiment 3
[0068] [Example 3] Synthesis of compound 12
[0069] Compound 12 (4.05 g, 65%) was obtained according to the synthesis method of compound 8.
[0070]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com