Preparation method of magnetic carbon nanocages
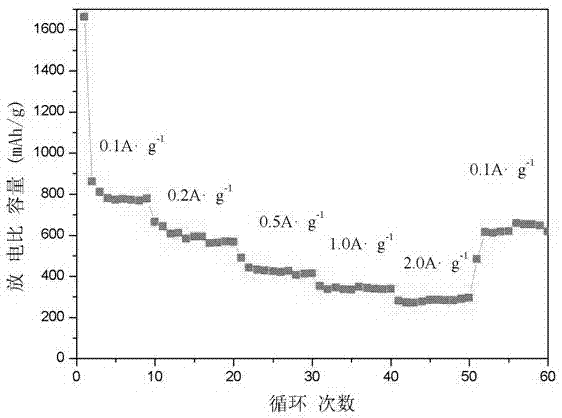
A nano-cage and magnetic carbon technology, which is applied in the direction of magnetic materials, magnetic objects, and the magnetism of inorganic materials, can solve the problems of unfavorable material entry and exit, inability to be used as nano-reactors, unfavorable drug loading, etc., to achieve low preparation costs and facilitate rapid Effect of transmission, excellent cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Preparation of Fe 3 o 4 ball:
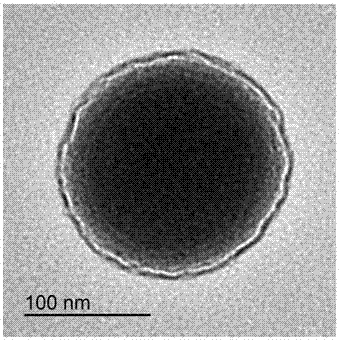
[0040] 3.6 g FeCl 3 ·6H 2 O, 90 ml of ethylene glycol, 10 ml of ethanol and 2.4 g of sodium acetate were mixed, mechanically stirred at room temperature for 30 min, the mixed solution was transferred to a reactor, and hydrothermally reacted at 200 °C for 10 h. After the reaction was completed, it was washed by centrifugation and dried to obtain 200 nm Fe 3 o 4 ball.
[0041] 2. Preparation of Fe 3 o 4 @SiO 2 @Cball:
[0042] Mix 60 ml of ethanol, 15 ml of deionized water and 3 ml of ammonia water, and mix 0.3 g Fe 3 o 4 The spheres are dispersed into the mixed solution to obtain Fe 3 o 4 of the mixture.
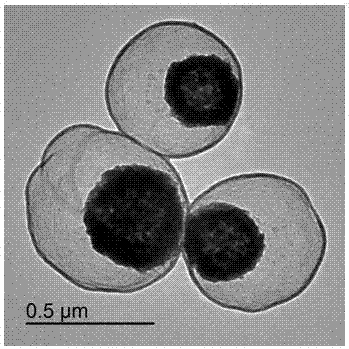
[0043] Add 0.3 g tetraethyl orthosilicate (TEOS) dropwise to Fe 3 o 4 In the mixed solution, stir mechanically at room temperature and react for 10 min to form a core-shell structure of Fe 3 o 4 @SiO 2 Nanospheres to obtain Fe with core-shell structure 3 o 4 @SiO 2 Nanosphere reaction system.
[0044] Add 0.5 ml...
Embodiment 2
[0050] 1. Preparation of Fe 3 o 4 ball:
[0051] 3.6 g FeCl 3 ·6H 2 O, 90 ml of ethylene glycol, 10 ml of ethanol and 2.4 g of sodium acetate were mixed, mechanically stirred at room temperature for 30 min, the mixed solution was transferred to a reactor, and hydrothermally reacted at 200 °C for 10 h. After the reaction was completed, it was washed by centrifugation and dried to obtain 200 nm Fe 3 o 4 ball.
[0052] 2. Preparation of Fe 3 o 4 @SiO 2 @Cball:
[0053] Mix 75 ml of ethanol, 30 ml of deionized water and 3 ml of ammonia water, and mix 0.3 g Fe 3 o 4 The spheres are dispersed into the mixed solution to obtain Fe 3 o 4 of the mixture.
[0054] Add 0.6 g tetraethyl orthosilicate (TEOS) dropwise to Fe 3 o 4 In the mixed solution, stir mechanically at room temperature and react for 15 min to form a core-shell structure of Fe 3 o 4 @SiO 2 Nanospheres to obtain Fe with core-shell structure 3 o 4 @SiO 2 Nanosphere reaction system.
[0055] Immediatel...
Embodiment 3
[0061] 1. Preparation of Fe 3 o 4 ball:
[0062] 3.6 g FeCl 3 ·6H 2 O, 90 ml of ethylene glycol, 10 ml of ethanol and 2.4 g of sodium acetate were mixed, mechanically stirred at room temperature for 30 min, the mixed solution was transferred to a reactor, and hydrothermally reacted at 200 °C for 10 h. After the reaction was completed, it was washed by centrifugation and dried to obtain 200 nm Fe 3 o 4 ball.
[0063] 2. Preparation of Fe 3 o 4 @SiO 2 @Cball:
[0064] Mix 150 ml of ethanol, 60 ml of deionized water and 3 ml of ammonia water, and mix 0.3 g Fe 3 o 4 The spheres are dispersed into the mixed solution to obtain Fe 3 o 4 of the mixture.
[0065] 3 g of tetraethyl orthosilicate (TEOS) was slowly added dropwise to Fe 3 o 4 In the mixed solution, stir mechanically at room temperature, react for 20 min, and form the core-shell structure of Fe 3 o 4 @SiO 2 Nanospheres to obtain Fe with core-shell structure 3 o 4 @SiO 2 Nanosphere reaction system.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com