Alkyl acetamidobenzotriazole derivative lubricating oil additive and preparation method thereof
A technology of benzotriazole and lubricating oil additives, which is applied in the field of alkylacetamidobenzotriazole derivative lubricating oil additives and its preparation, can solve problems such as environment and health, and achieve good Extreme pressure and anti-wear properties, mild reaction conditions, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
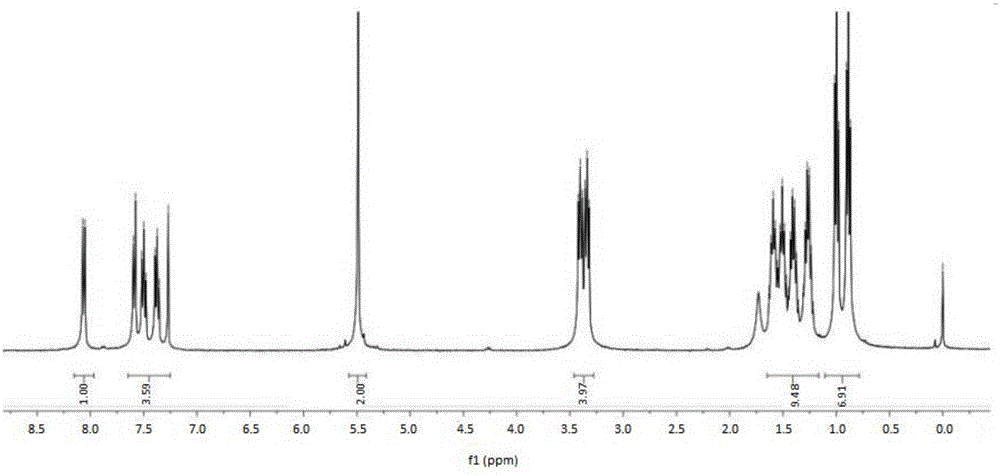
[0036] Add 0.1mol (12.9g) di-n-butylamine, 0.1mol (12.2g) 4-dimethylaminopyridine (DMAP) and 75mL chloroform to a 250mL three-necked flask, add dropwise under ice bath (0℃~5℃) Chloroacetyl chloride 0.1mol (11.3g), stirred and reacted for 3h. After the reaction, the reaction solution was washed three times with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was rotary evaporated to remove the solvent to obtain N,N-dibutyl-2-chloroacetamide.
[0037] Add 0.1 mol (11.9 g) benzotriazole, 0.1 mol (4.0 g) NaOH, 100 mL ethanol to a 250 mL three-necked flask. At room temperature, add N,N-dibutyl-2-chloride dissolved in 20 mL tetrahydrofuran dropwise Acetamide, after stirring for 1.5 hours, the temperature was raised and refluxed for 3.5 hours. After the reaction, it was cooled to room temperature, the insoluble matter was removed by filtration, the solvent was removed from the filtrate, and the silica gel column chromatography was used for separation...
Embodiment 2
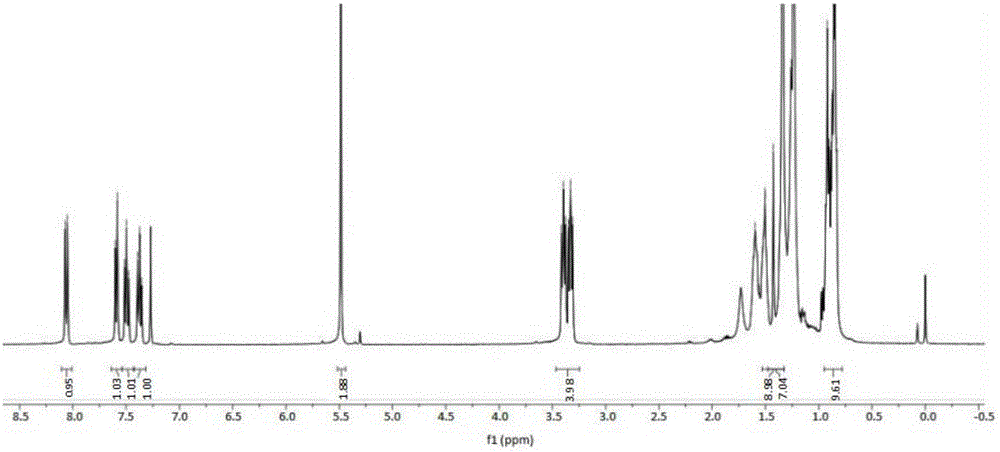
[0039] Add 0.1mol (18.5g) di-n-hexylamine, 0.1mol (12.2g) 4-dimethylaminopyridine (DMAP), 75mL chloroform to a 250mL three-necked flask, add chlorine dropwise under ice bath (0℃~5℃) Acetyl chloride 0.1mol (11.3g), stirred and reacted for 3h. After the reaction, the reaction solution was washed three times with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was rotary evaporated to remove the solvent to obtain N,N-dihexyl-2-chloroacetamide.
[0040] Add 0.1 mol (11.9 g) benzotriazole, 0.1 mol (4.0 g) NaOH, and 100 mL ethanol to a 250 mL three-necked flask. At room temperature, add N,N-dihexyl-2-chloroethyl dissolved in 20 mL tetrahydrofuran dropwise After stirring for 1.5h, the amide was heated and refluxed for 3.5h. After the reaction, it was cooled to room temperature, the insoluble matter was removed by filtration, the solvent was removed from the filtrate, and the silica gel column chromatography was used for separation and purification to...
Embodiment 3
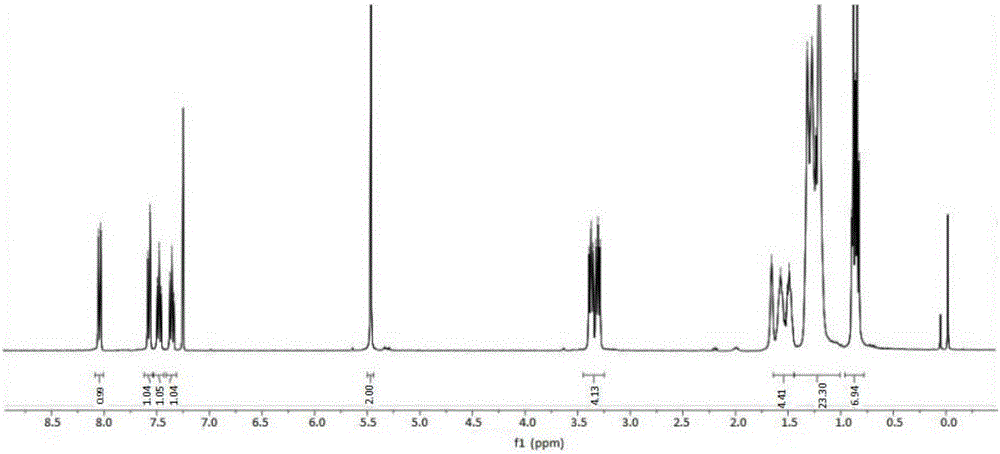
[0042] Add 0.1mol (24.1g) di-n-octylamine, 0.1mol (12.2g) 4-dimethylaminopyridine (DMAP), 75mL chloroform into a 250mL three-necked flask, dropwise under ice bath (0℃~5℃) 0.1mol (11.3g) of chloroacetyl chloride was added, and the reaction was stirred for 3h. After the completion of the reaction, the reaction solution was washed three times with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was rotary evaporated to remove the solvent to obtain N,N-dioctyl-2-chloroacetamide.
[0043] Add 0.1 mol (11.9 g) benzotriazole, 0.1 mol (4.0 g) NaOH, and 100 mL ethanol to a 250 mL three-necked flask. Add N,N-dioctyl-2-chloride dissolved in 20 mL tetrahydrofuran at room temperature. Acetamide, after stirring for 1.5 hours, the temperature was raised and refluxed for 3.5 hours. After the reaction, it was cooled to room temperature, the insoluble matter was removed by filtration, the solvent was removed from the filtrate, and the silica gel column chromato...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Onset thermal decomposition temperature | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com