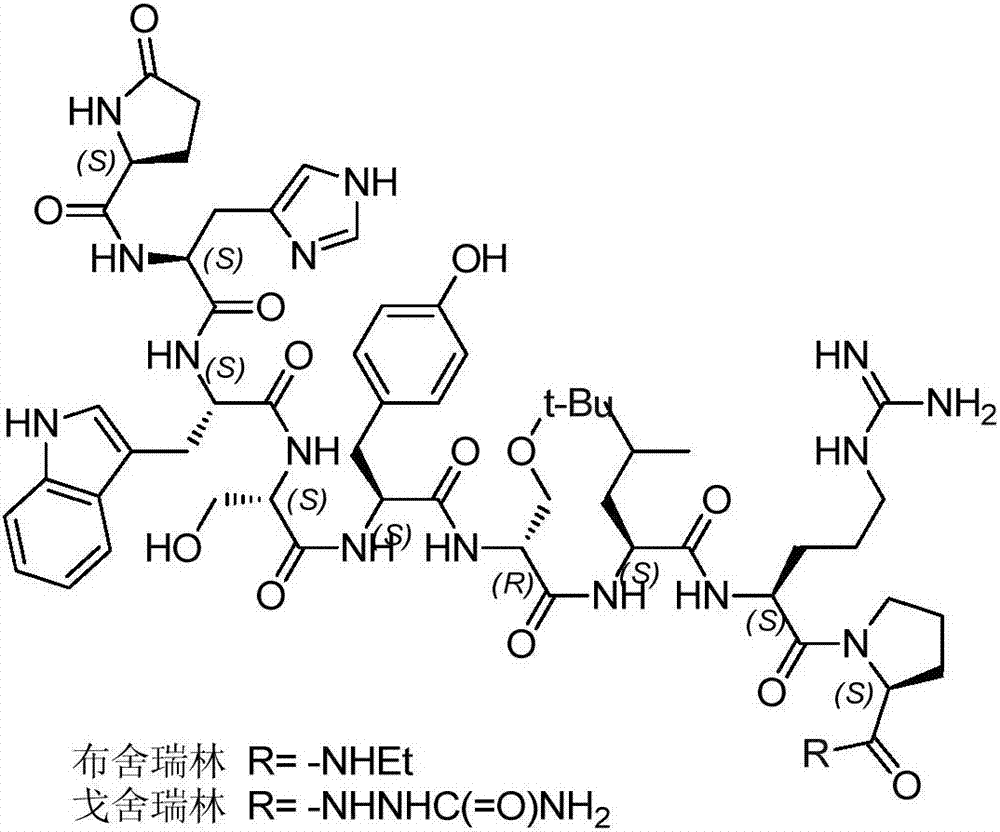
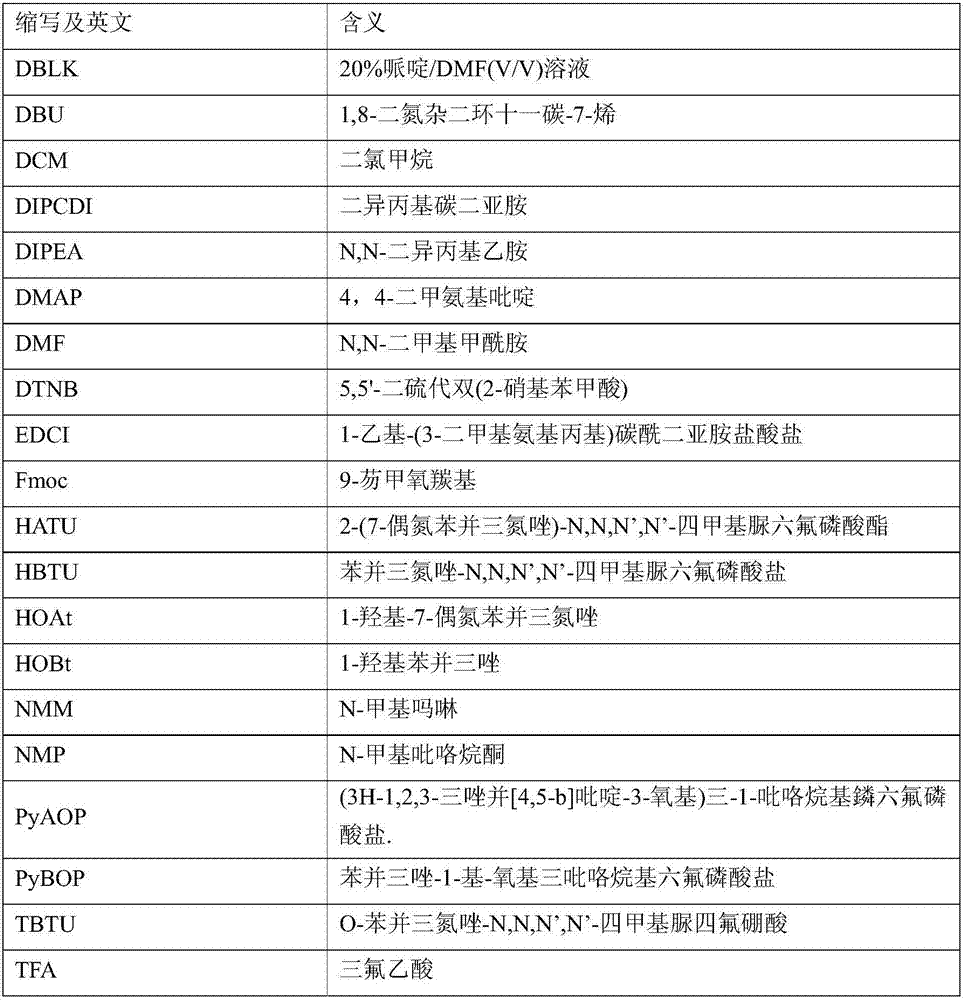
Preparation method of buserelin or goserelin
A technology for goserelin and protection of goserelin, which is applied in the field of preparation of buserelin or goserelin, can solve the problems of uncertainty, increase the impurity content, and is difficult to remove, and achieves mild reaction conditions and easy operation. Simple, selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of embodiment 1 fully protected peptide resin
[0031] Weigh 55.0 g (50 mmol) of 2-CTC resin with a substitution degree of 0.91 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes. Weigh 33.7g (100mmol) Fmoc-Pro-OH, add appropriate amount of DMF to dissolve, add 12.9g (100mmol) DIPEA under ice-water bath, stir for 4-6 minutes, then add to the resin, after coupling reaction for 5 minutes, add more 12.9g (100mmol) DIPEA, after coupling reaction 1 hour, add methanol 55ml in the reaction solution, after blocking reaction 20 minutes, extract reaction solution, DMF washes resin three times, obtains Fmoc-Pro-CTC resin.
[0032] Add DBLK for deprotection for 5+7 minutes, and wash the resin 6 times with DMF. Weigh 66.2g (150mmol) Fmoc-Arg (NO 2 )-OH and 22.5g (165mmol) HOAt were dissolved with an appropriate amount of DMF. Add 22.6g (180mmol) DICPDI in an ice-water bath, stir for 4 to 6 minutes, add to...
Embodiment 2
[0035] The synthesis of embodiment 2 fully protected peptide resins
[0036]Weigh 48.4 g (30 mmol) of 2-CTC resin with a substitution degree of 0.62 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes. Weigh 20.2g (60mmol) Fmoc-Pro-OH, add appropriate amount of DMF to dissolve, add 7.7g (60mmol) DIPEA under ice-water bath, stir for 4-6 minutes, then add to the resin, after coupling reaction for 5 minutes, add more 7.7g (60mmol) DIPEA, after coupling reaction 1 hour, add methanol 45ml in the reaction solution, after blocking reaction 20 minutes, extract reaction solution, DMF washes resin three times, obtains Fmoc-Pro-CTC resin.
[0037] Add DBLK for deprotection for 5+7 minutes, and wash the resin 6 times with DMF. Weigh 39.7g (90mmol) Fmoc-Arg (NO 2 )-OH and 13.5g (99mmol) HOAt were dissolved with an appropriate amount of DMF. Add 13.6g (108mmol) DICPDI in an ice-water bath, stir for 4-6 minutes, add to the r...
Embodiment 3
[0040] Example 3 Cleavage Preparation of Fully Protected Crude Peptide
[0041] 101.5 g of the fully protected peptide resin obtained in Example 2 was added into a 2000 ml round bottom flask. Add pre-prepared TFA:DCM=2:98 (V:V) 1015ml, react at room temperature for 2.5 hours, filter the resin, and collect the filtrate. The resin was washed with a small amount of DCM, and the filtrates were combined. The filtrate was slowly added to 10.15 L of glacial ether for precipitation, centrifuged, washed twice with ether, and dried under reduced pressure to obtain 50.2 g of a fully protected crude peptide with an HPLC purity of 93.9%. The theoretical yield is 55.0 g, and the weight yield is 91.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com