Method for coating nickel-cobalt lithium manganate and obtained nickel-cobalt lithium manganate material
A nickel-cobalt lithium manganese oxide coating technology, which is applied in the field of lithium-ion battery electrode materials, can solve the problems of reduced migration rate, interface impedance, cycle performance, and battery impedance, so as to increase Li+ migration rate and improve electrochemical performance. , The effect of small material phase impedance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
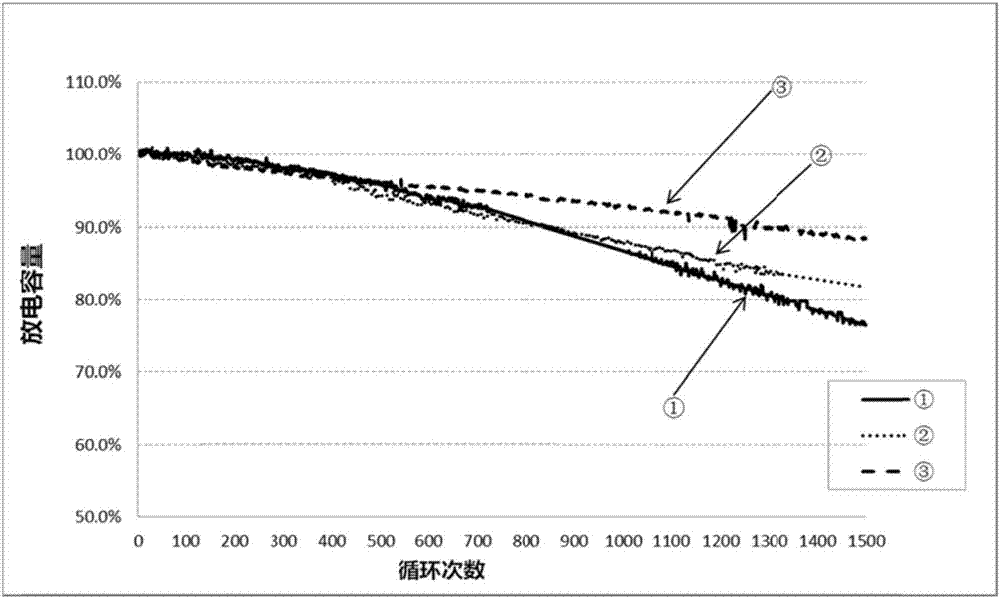
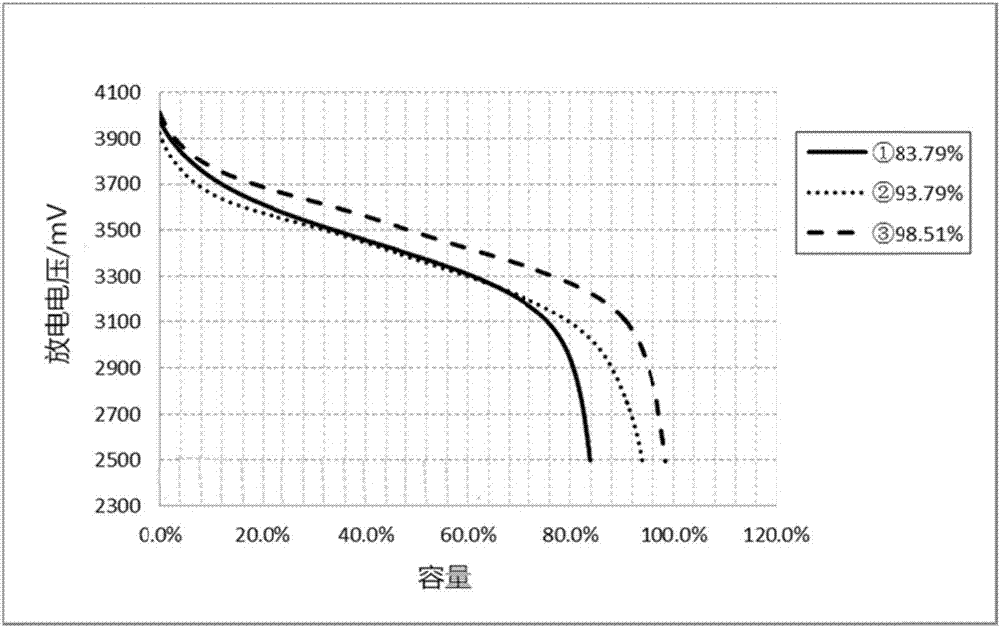
Image
Examples
Embodiment 1
[0026] A method for coating nickel-cobalt lithium manganese oxide, prepared by the following steps:
[0027] a) Mix Ni in a certain proportion 1-x-y mn x co y (OH) 2 precursors and lithium salts to make Ni 1-x-y mn x co y (OH) 2 The molar ratio with Li is 1:0.9. When mixing, add the same volume of deionized water as the precursor and lithium salt. The mixture is sintered at 500°C for 5 hours, and then pulverized after sintering to obtain D 50 5~12μm powder; Ni 1-x-y mn x co y (OH) 2 In the precursor x=0.1, y=0.1, the precursor Ni 1-x-y mn x Ci y (OH) 2 The particle size is D 50 = 5 μm;
[0028] b) mix the powder obtained by step a with Ni 1-a-b mn a co b (OH) 2 The precursor and the lithium salt are mixed in a certain proportion, and the same volume of deionized water as the precursor and the lithium salt is added during mixing. Coated nickel cobalt lithium manganese oxide material; wherein Ni 1-a-b mn a co b (OH) 2 The molar ratio with Li is 1:0.9; Ni...
Embodiment 2
[0032] A method for coating nickel-cobalt lithium manganese oxide, prepared by the following steps:
[0033] a) Mix Ni in a certain proportion 1-x-y mn x co y (OH) 2 precursors and lithium salts to make Ni 1-x-y mn x co y (OH) 2 The molar ratio with Li is 1:1. When mixing, add the precursor and deionized water twice the volume of lithium salt. The mixture is sintered at 750°C for 10 hours, and then pulverized after sintering to obtain D 50 8μm powder; Ni 1-x-y mn x co y (OH) 2 In the precursor x=0.15, y=0.15, the precursor Ni 1-x-y mn x co y (OH) 2 The particle size is D 50 = 7μm;
[0034] b) mix the powder obtained by step a with Ni 1-a-b mn a co b (OH) 2 The precursor and the lithium salt were mixed in a certain proportion. When mixing, deionized water twice the volume of the precursor and the lithium salt was added. After the mixture was fully mixed, the liquid mixture was spray-dried and sintered in an air atmosphere at 850°C for 10 hours. Coated nicke...
Embodiment 3
[0038] A method for coating nickel-cobalt lithium manganese oxide, prepared by the following steps:
[0039] a) Mix Ni in a certain proportion 1-x-y mn x co y (OH) 2 precursors and lithium salts to make Ni 1-x-y mn x co y (OH) 2 The molar ratio with Li is 1:1. When mixing, add the precursor and deionized water twice the volume of lithium salt. The mixture is sintered at 750°C for 10 hours, and then pulverized after sintering to obtain D 50 8μm powder; Ni 1-x-y mn x co y (OH) 2 In the precursor x=0.15, y=0.15, the precursor Ni 1-x-y mn x co y (OH) 2 The particle size is D 50 = 7μm;
[0040] b) mix the powder obtained by step a with Ni 1-a-b mn a co b (OH) 2 The precursor and the lithium salt were mixed in a certain proportion. When mixing, deionized water twice the volume of the precursor and the lithium salt was added. After the mixture was fully mixed, the liquid mixture was spray-dried and sintered in an air atmosphere at 850°C for 10 hours. Coated nicke...
PUM
| Property | Measurement | Unit |
|---|---|---|
| D50 | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com