Macromolecular polyaspartic acid ester and preparation method thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
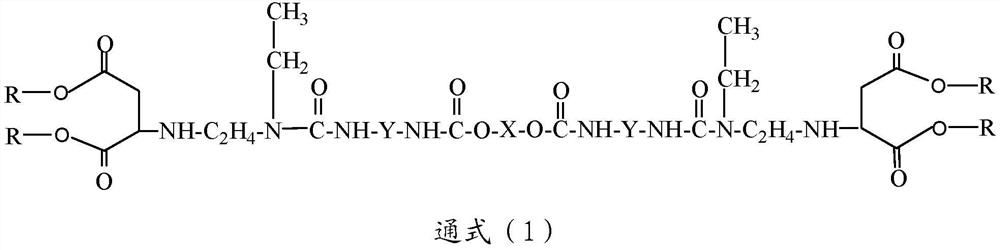
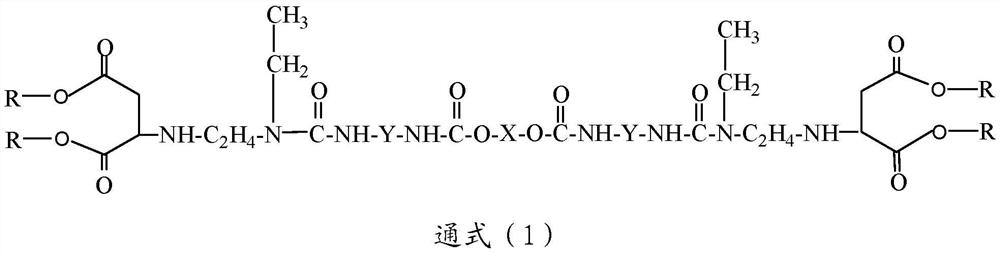
[0049] In addition, the present invention also provides a preparation method of macromolecular polyaspartic acid ester, said method comprising the following steps:
[0050] a) reacting polyether polyol and polyisocyanate to obtain a polyether prepolymer terminated at both ends by said polyisocyanate;
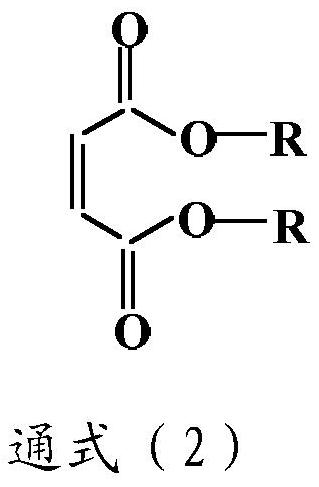
[0051] b) reacting equimolar N-ethylethylenediamine and a dicarboxylic acid ester to obtain N-ethylethylenediamine capped at one end by said dicarboxylic acid ester; and
[0052] c) mixing and heating the polyether prepolymer obtained in step a) and the N-ethylethylenediamine terminated at one end by the dicarboxylic acid ester obtained in step b) to obtain the The macromolecular polyaspartic acid ester described above.
[0053] According to some embodiments of the present invention, the polyether polyol is selected from polyethylene oxide polyols, polypropylene oxide polyols and polytetrahydrofuran polyols having a hydroxyl functionality of 2 or 3 and a number average molecula...
Embodiment 1
[0086] Add 500 grams of polytetrahydrofuran with a hydroxyl functionality of 2 and a molecular weight of 2000 and 112 grams of isophorone diisocyanate in a four-necked flask equipped with a stirrer, a thermometer, a nitrogen inlet, and a reflux device, and feed nitrogen into the mixture. Heating to 90° C. and reacting for 3 hours to obtain a polytetrahydrofuran prepolymer whose two ends are blocked by isophorone diisocyanate.
[0087] Add 44 grams of N-ethylethylenediamine in a four-necked flask equipped with a stirrer, a thermometer, a nitrogen inlet and a reflux device, feed nitrogen, and slowly dissolve 86 grams of diethyl maleate under cooling conditions in a water bath at 0 ° C. Add it dropwise, and then stir at room temperature for 2.0 hours to obtain N-ethylethylenediamine with one end capped by diethyl maleate. Slowly drop 612 grams of the polytetrahydrofuran whose both ends are blocked by isophorone diisocyanate prepared above into it under the condition of cooling in...
Embodiment 2
[0089] Add 1000 grams of polytetrahydrofuran and 84 grams of hexamethylene diisocyanate with a hydroxyl functionality of 2 and a molecular weight of 4000 and 84 grams of hexamethylene diisocyanate in a four-necked flask equipped with a stirrer, a thermometer, a nitrogen inlet, and a reflux device. Heating to 90° C. for 2 hours to obtain a polyether prepolymer whose two ends are blocked by hexamethylene diisocyanate.
[0090] Add 44 grams of N-ethylethylenediamine in the four-necked flask equipped with stirrer, thermometer, nitrogen inlet and reflux device, pass into nitrogen, slowly dissolve 114 grams of dibutyl maleate under 0 ℃ water bath cooling condition Add it dropwise, and then stir at room temperature for 2 hours to obtain N-ethylethylenediamine whose end is capped with diethyl maleate. Slowly drop 1112 grams of the polytetrahydrofuran whose both ends are capped by hexamethylene diisocyanate prepared above into it under the condition of cooling in a water bath, heat to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com