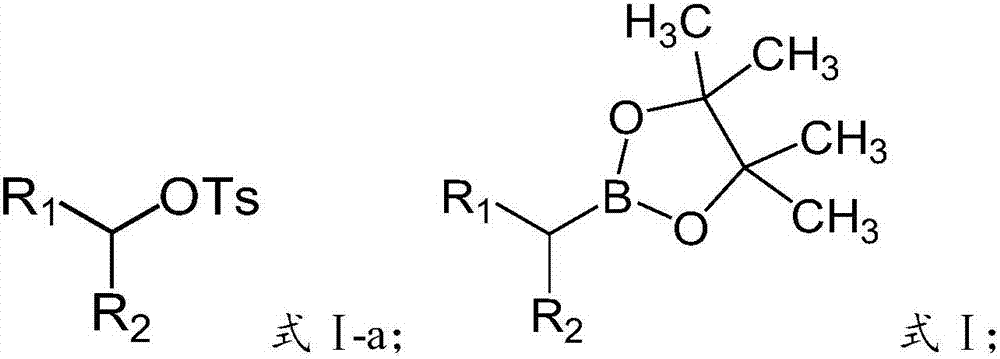
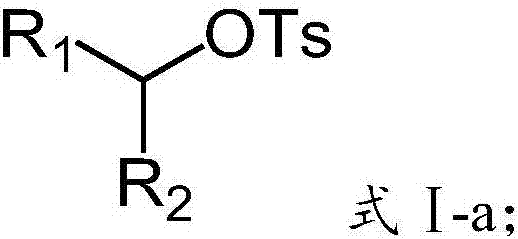
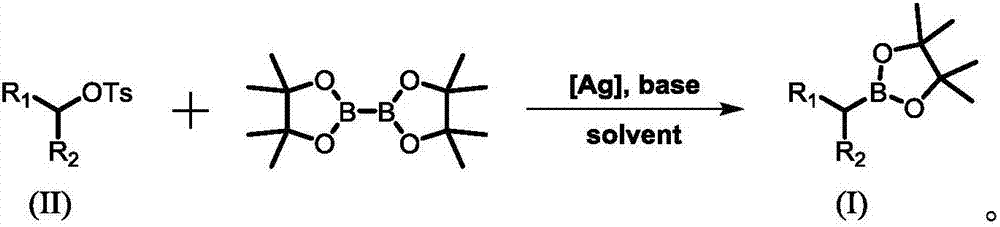
Preparation method of alkyl borate
A technology of alkyl borate and diboronic acid pinacol ester, which is applied in the field of preparation of alkyl borate, can solve the problems of high catalyst cost and few convenient and universal methods, and achieve good functional group compatibility, Wide range of raw material sources and simple feeding methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The reaction formula of this embodiment is as follows:
[0042]
[0043] (1) Under air, silver pentafluoropropionate (10mol%) and lithium tert-butoxide (2eq) were added to a pressure-resistant sealed reaction tube containing magnetons. Under the protection of argon, add 1 mL of 1,4-dioxane to the reaction tube, stir at room temperature for 20 minutes, then add pinacol diboronate (1.25 eq) and 4-benzene Add butyl-2-p-toluenesulfonate (0.25mmol) into the reaction liquid, stopper the stopper, place in a 60°C oil bath and stir for 24 hours.
[0044](2) Cool the material obtained in step (1) to room temperature, add ethyl acetate and mix thoroughly, filter the solid residue with a short silica gel column, and keep the organic phase.
[0045] (3) Spin dry the solvent in the organic phase obtained in step (2) to obtain a crude product, and then purify the crude product with a silica gel column. The eluent is a mixed solution of petroleum ether and ethyl acetate, and the r...
Embodiment 2~14
[0048] The compounds in Table 1 were prepared according to the method of Example 1.
[0049] Table 1 Example 1~14 product structure and characterization data
[0050]
[0051]
[0052]
Embodiment 15~20
[0054] The raw materials of Example 1 were used to replace the catalyst and alkali to prepare alkyl borates, and the yields are shown in Table 2.
[0055] Table 2 embodiment 15~20 catalyst, alkali and productive rate summary
[0056]
[0057]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com