Preparation method of graphite phase carbon nitride@MOF nano-crystals and application thereof
A technology of graphitic carbon nitride and nanocrystals, which is applied in the direction of nanotechnology, nanotechnology, nitrogen and non-metallic compounds, etc., can solve the problems that hinder the production and application of materials, increase the preparation cost, etc., and achieve long preparation cycle and low cost , Increase the effect of economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1. Preparation of Ligand L
[0036] Under stirring conditions, mix 0.10 mol of 1,2-ethylenediamine and 0.20 mol of 3-pyridinecarboxylic acid, heat fractionation, and maintain the temperature at the top of the fractionation column at 103-105 °C. When the temperature at the top of the fractionation column drops, it indicates that the reaction has been completed , the mixture was cooled to 10-20°C, filtered with suction, washed three times with ethanol, and recrystallized with ethanol to obtain ligand L with a yield of 65%;
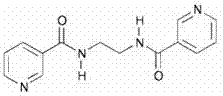
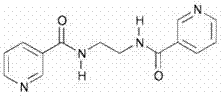
[0037] Ligand L, the structural formula is as follows:
[0038]
Embodiment 2
[0039] Example 2 A preparation method of graphitic carbon nitride @MOF nanocrystals
[0040] 0.058 g Co(NO 3 ) 2 ﹒ 6H 2 Dissolve O in 0.5 mL of water, add graphitic carbon nitride 0.01 g graphitic carbon nitride g-C 3 N 4 , making g-C 3 N 4 @Co(II) aqueous solution;
[0041] Dissolve 0.03 g of ligand L in 0.5 mL of N,N-dimethylacetamide DMA to prepare a DMA solution of ligand L;
[0042] 0.025 g terephthalic acid H 2 BDC, 0.020 g NaOH were dissolved in 0.5 mL water to prepare H 2 BDC alkaline aqueous solution;
[0043] will g-C 3 N 4 @Co(II) aqueous solution and H 2 After blending the BDC alkaline aqueous solution at room temperature, add the DMA solution of Ligand L and shake and mix well. After 20 seconds, a pink gel is obtained. The gel is aged at 85 °C for 24 h, and the volume ratio of 1:1 with ethanol and water After washing and centrifuging for 3 times, and vacuum drying at 70°C, the graphitic carbon nitride @MOF nanocrystals were prepared.
Embodiment 3
[0044] Example 3 A preparation method of graphitic carbon nitride @MOF nanocrystals
[0045] 0.058 g Co(NO 3 ) 2 ﹒ 6H 2 Dissolve O in 0.75 mL of water, add graphitic carbon nitride 0.015 g graphitic carbon nitride g-C 3 N 4 , making g-C 3 N 4 @Co(II) aqueous solution;
[0046] Dissolve 0.040 g of ligand L in 0.75 mL of N,N-dimethylacetamide DMA to prepare a DMA solution of ligand L;
[0047] 0.030 g terephthalic acid H 2 BDC, 0.025 g NaOH was dissolved in 0.75 mL water to prepare H 2 BDC alkaline aqueous solution;
[0048] will g-C 3 N 4 @Co(II) aqueous solution and H 2 After blending the BDC alkaline aqueous solution at room temperature, add the DMA solution of Ligand L and shake and mix well. After 70 seconds, a pink gel is obtained. The gel is aged at 85 ℃ for 24 h, and the ethanol and water with a volume ratio of 1:1 are used respectively. After washing and centrifuging for 3 times, and vacuum drying at 70°C, the graphitic carbon nitride @MOF nanocrystals wer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com