Gold nanocluster-based 6-mercaptopurine detection method and kit
A technology of gold nano-clusters and detection kits, which is applied in the field of analytical chemistry and nanometers, can solve the problems of complex measurement steps, long response time, and poor reproducibility, and achieve the effects of easy promotion and use, short detection time, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
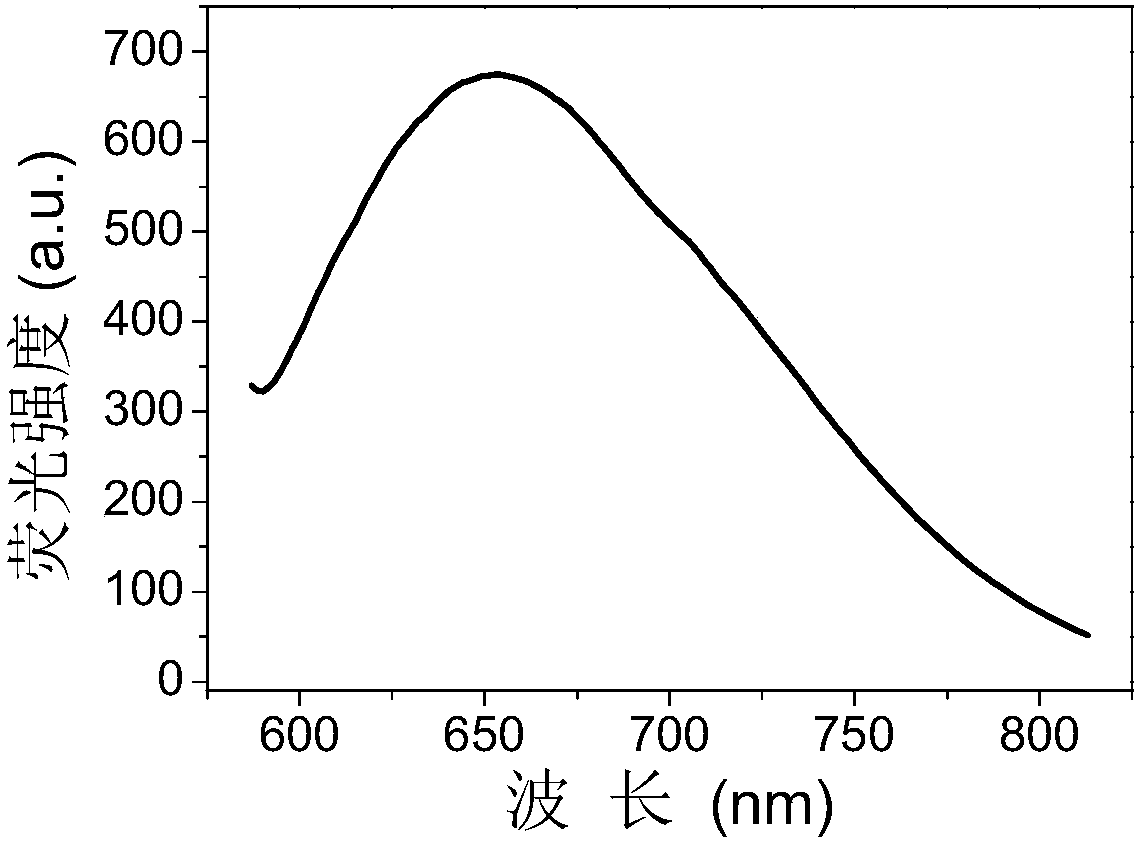
[0038] 1 mL of 0.4 mol / L sodium hydroxide solution and 1.6 mL of 20 mg / mL chloroauric acid were mixed in advance, and then 7.4 mL of 50 mg / mL carboxylated chitosan solution and 10 mL of 0.1 mol / L dithiothreitol solution, shake well and place in a water bath at 37°C for a constant temperature reaction for 8 hours to obtain a carboxylated chitosan-dithiothreitol-gold nanocluster solution. The reaction solution changed from light yellow to colorless. The reacted gold nanocluster solution was dialyzed in double distilled water for 24 h with a dialysis bag with a molecular weight cut off of 3500 to obtain a purified carboxylated chitosan-dithiothreitol-gold nanocluster solution. The resulting carboxylated chitosan-dithiothreitol-gold nanoclusters are strongly fluorescent with a maximum emission wavelength of 650 nm (see figure 1 ).
Embodiment 2
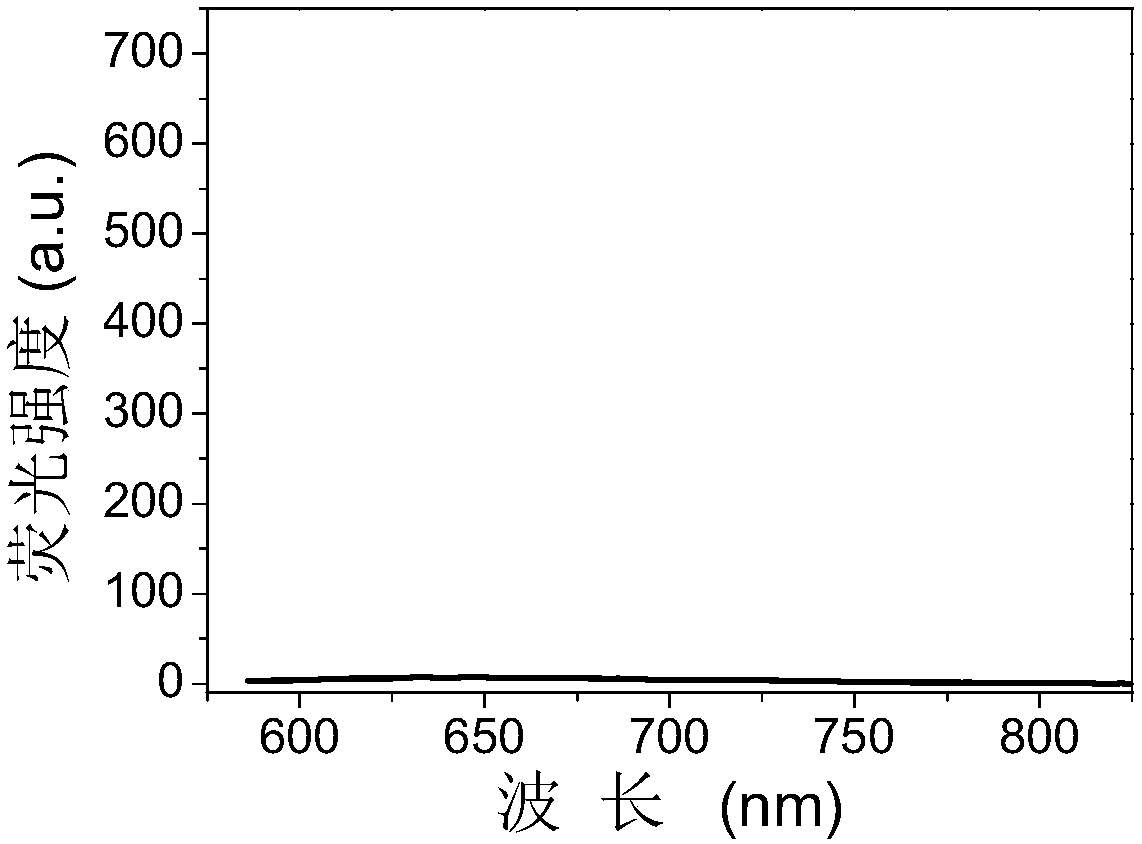
[0040] Take 0.1 mL of the 1.6 mg / mL gold nanocluster solution prepared in Example 1 and add it to 1.9 mL of phosphate buffer (20 mmol / L, pH=7.4) containing 1 mmol / L 6-mercaptopurine, and mix well After reacting at room temperature for 10 min, the emitted light intensity value of the system at 650 nm was significantly lower than that of Example 1 (see figure 2 ).
Embodiment 3
[0042] Take 0.1 mL of the 1.6 mg / mL gold nanocluster solution prepared in Example 1 and add it to 1.9 mL of phosphate buffer (20 mmol / L, pH=7.4) containing 1 mmol / L 6-mercaptopurine, and mix well Then react at room temperature for 10 min. At the same time, a group of blank control groups without 6-mercaptopurine was set up. After the reaction was completed, the color change was observed under ultraviolet light. Such as image 3 As shown, the red fluorescence of gold nanoclusters was almost completely quenched after adding 6-mercaptopurine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com