Method for rapidly preparing cobalt oxide
A cobalt oxide, fast technology, applied in the direction of cobalt oxide/cobalt hydroxide, can solve the problems of increasing the consumption of chemical materials, difficult to improve the cobalt leaching rate, increasing production costs, etc., to reduce chemical precipitation and removal of impurities and organic extraction depth. The effect of impurity and other processes and technical conditions is stable and reliable, and the cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
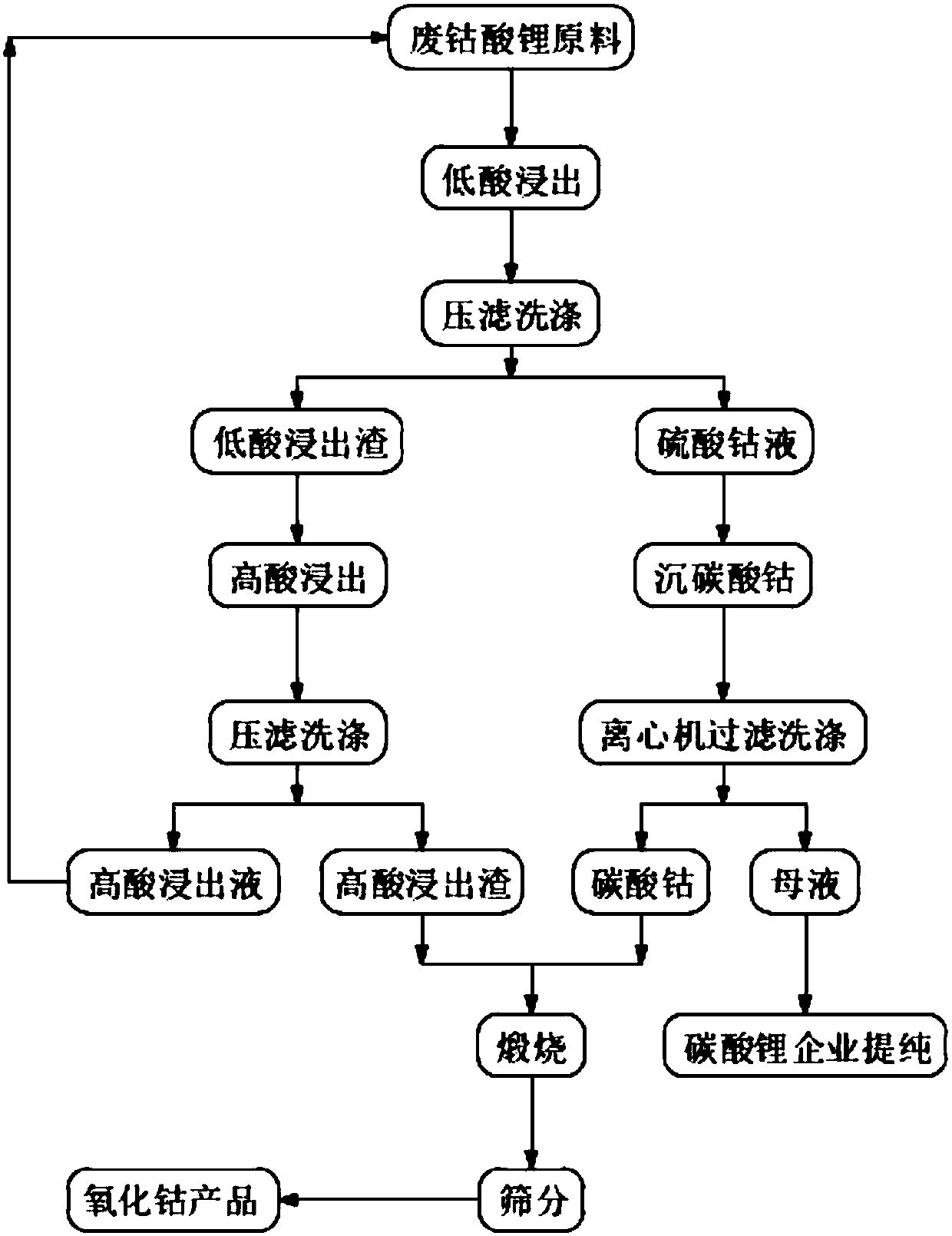
[0036] 1) Mixing: Put the waste lithium cobaltate raw material and pure water into a leaching tank with a mechanical stirring blade at a volume ratio of 1:7.5 and stir and mix for 1 hour to obtain a mixed slurry. The raw material composition of waste lithium cobaltate; Co55.8% , Ni0.06%, Mn0.2%, Ca0.08%, Mg0.06%, Li6.5%;
[0037] 2) Low-acid leaching: add inorganic acid (sulfuric acid) into the mixed slurry through the flow guide tube at a flow rate of 80L / min, keep the stirring speed at 100r / min, and the initial concentration of sulfuric acid at 3N, open the steam valve at the same time, and start to heat up , reacted for 4h under the condition of controlling the temperature at 90°C, sampled and analyzed, when the pH value in the solution=1, the reaction was stopped; liquid-solid separation was carried out through a filter press to obtain the main component of the leaching solution (cobalt sulfate) (g / L); Co22, Ni0.01, Mn0.02, Ca0.03, Mg0.03, Li7.5 and low acid leaching slag...
Embodiment 2
[0042] 1) Mixing: put the waste lithium cobaltate raw material and purified water into a leaching tank with a mechanical stirring blade at a volume ratio of 1:7, and stir and mix for 1 hour to obtain a mixed slurry. Raw material composition of waste lithium cobaltate: Co55.8%, Ni0.06%, Mn0.2%, Ca0.08%, Mg0.06%, Li6.5%;
[0043] 2) Low-acid leaching: Add inorganic acid (sulfuric acid) into the mixed slurry through the flow guide tube at a flow rate of 50L / min, keep the stirring speed at 75r / min, and the initial concentration of sulfuric acid at 2.5N. At the same time, open the steam valve and start Heating up, reacting for 3.5h under the condition of controlling the temperature at 80° C., sampling and analyzing, when the pH value=1 in the solution, then stop the reaction; carry out liquid-solid separation through a filter press to obtain the main component of the leaching solution (cobalt sulfate) ( g / L): Co20.5, Ni0.03, Mn0.03, Ca0.02, Mg0.04, Li7.1 and the main components of ...
Embodiment 3
[0048] 1) Mixing: Put the waste lithium cobaltate raw material: pure water into the leaching tank with mechanical stirring blades at a volume ratio of 1:6.5 and stir and mix for 1 hour to obtain a mixed slurry. Raw material composition of waste lithium cobaltate: Co55.8%, Ni0.06%, Mn0.2%, Ca0.08%, Mg0.06%, Li6.5%;
[0049] 2) Low-acid leaching: Add inorganic acid (sulfuric acid) into the mixed slurry through the flow guide tube at a flow rate of 55L / min, keep the stirring speed at 80r / min, and the initial concentration of sulfuric acid at 2.5N. At the same time, open the steam valve and start Heating up, reacting 4h under the condition of controlling temperature 86 ℃, sampling analysis, when the pH value=1.5 in the solution, then stop reaction;, carry out liquid-solid separation through filter press, obtain leaching solution (cobalt sulfate) main component ( g / L); Co20, Ni0.02, Mn0.01, Ca0.02, Mg0.03, Li6.8 and the main components of low-acid leaching slag (g / L) Co60.5%, Li0.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com