Continuous chromatography separation and purification method for lincomycin
A continuous chromatography and lincomycin technology, which is applied in the field of separation and purification of the antibacterial drug lincomycin, can solve the problems of high impurity content, long production process time, low content of lincomycin hydrochloride, etc., and achieve product composition and The effect of maintaining stable concentration and reducing the amount of resin used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Step 1, applying the lincomycin fermentation filtrate to a continuous chromatographic column to obtain an eluate;
[0064] In step 2, the eluate is concentrated, crystallized and dried to obtain pure lincomycin.
[0065] Wherein, the continuous chromatographic column mentioned in step 1, the resin is selected from: macroporous adsorption resin HZ-802, the resin particle diameter is 50-100 mesh, and the uniformity is above 95%.
[0066] Wherein, the process of concentrating and drying the eluate described in step 2 is as follows: concentrating under reduced pressure, the vacuum degree is 0.02-0.15Mpa, the operating temperature is 35-90°C, and concentrating until the mass concentration of lincomycin is 25%. ~70% to obtain a concentrated solution; the concentrated solution is crystallized with acetone to obtain a solid.
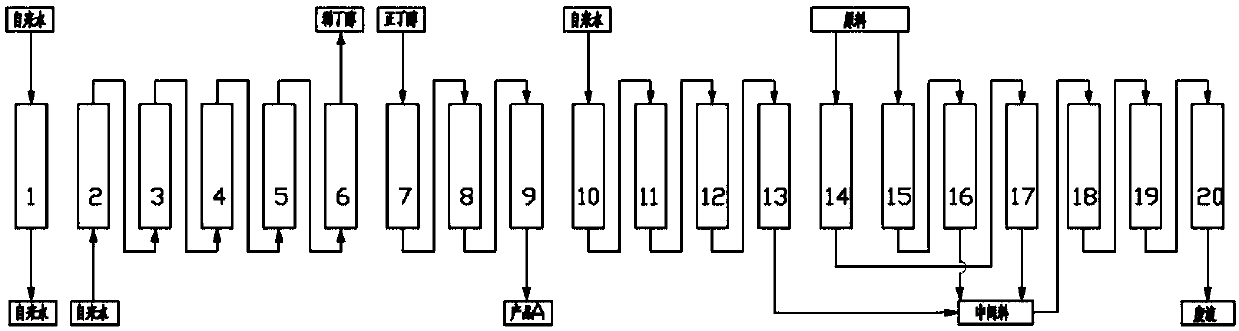
[0067] Wherein, the continuous chromatography is selected from: a disc conveying continuous chromatography separation system or a simulated moving bed c...
Embodiment 2
[0093] Proceed as follows:
[0094] Step 1, applying the lincomycin fermentation filtrate to a continuous chromatographic column to obtain an eluent;
[0095] In step 2, the eluate is concentrated, crystallized and dried to obtain pure lincomycin.
[0096] Wherein, the continuous chromatographic column mentioned in step 1, the resin is selected from: macroporous adsorption resin HZ-802, the resin particle diameter is 50-100 mesh, and the uniformity is above 95%.
[0097] Wherein, the process of concentrating and drying the eluate described in step 2 is as follows: concentrating under reduced pressure, the vacuum degree is 0.02-0.15Mpa, the operating temperature is 35-90°C, and concentrating until the mass concentration of lincomycin is 25%. ~70% to obtain a concentrated solution; the concentrated solution is crystallized with acetone to obtain a solid.
[0098] Wherein, the continuous chromatography is selected from: a disc conveying continuous chromatography separation syst...
Embodiment 3
[0134] Embodiment 3, the comparison of purification method of the present invention and existing purification method
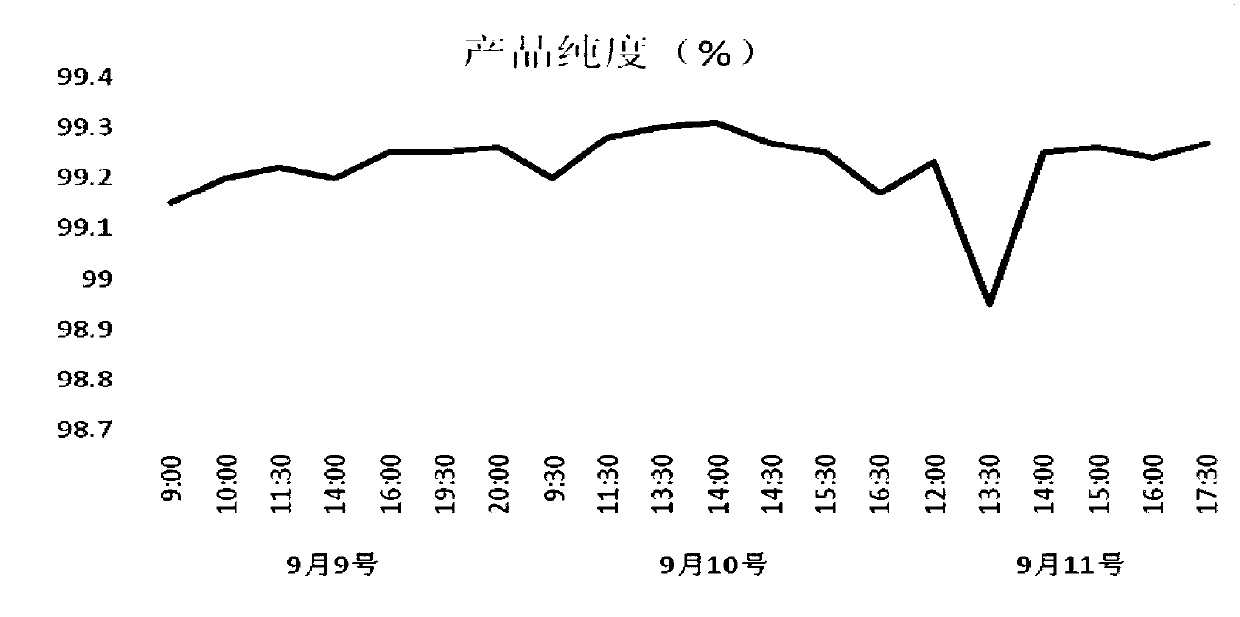
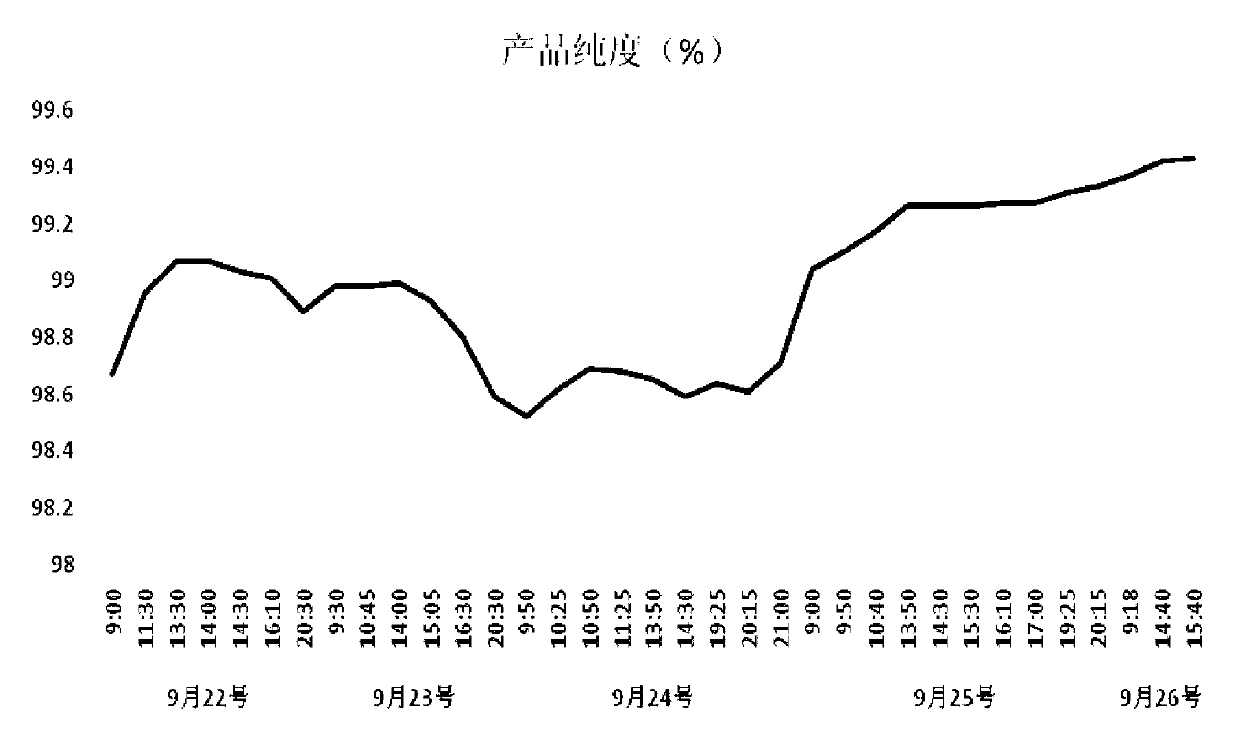
[0135] In order to investigate the best preparation method of lincomycin, the preparation method of the present invention was compared with the existing technology, wherein: scheme 1: the fermentation broth is filtered to remove the mycelium after acidification treatment, and the filtrate is adjusted to pH to 11. Add butanol to it for extraction and refining, add HCL solution for back extraction, concentrate under reduced pressure, adsorb on activated carbon, crystallize, and dry to obtain lincomycin hydrochloride raw material drug. Scheme 2: Purification method of the present invention (Example 2). The result shows that the purity index of lincomycin purified by the purification method of the present invention is obviously better than that of the existing purification method.
[0136]
[0137]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com