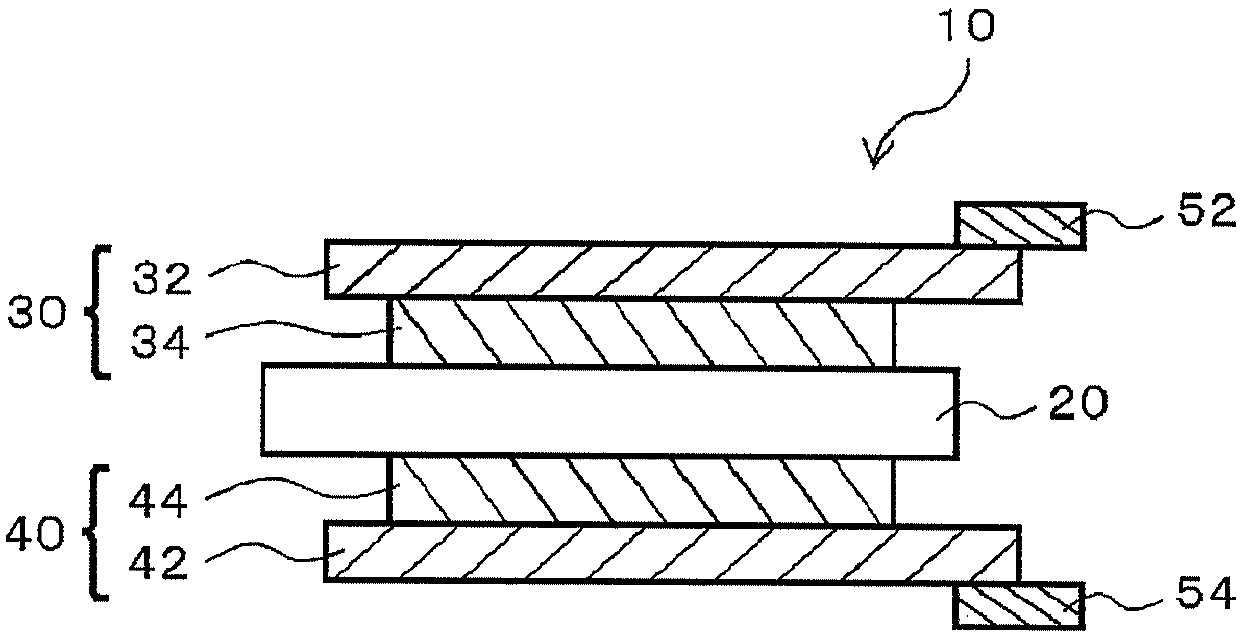
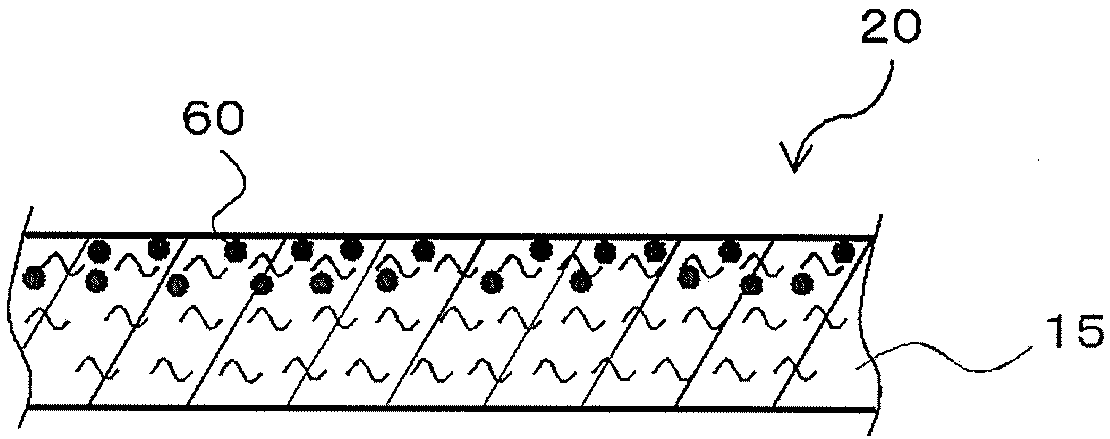
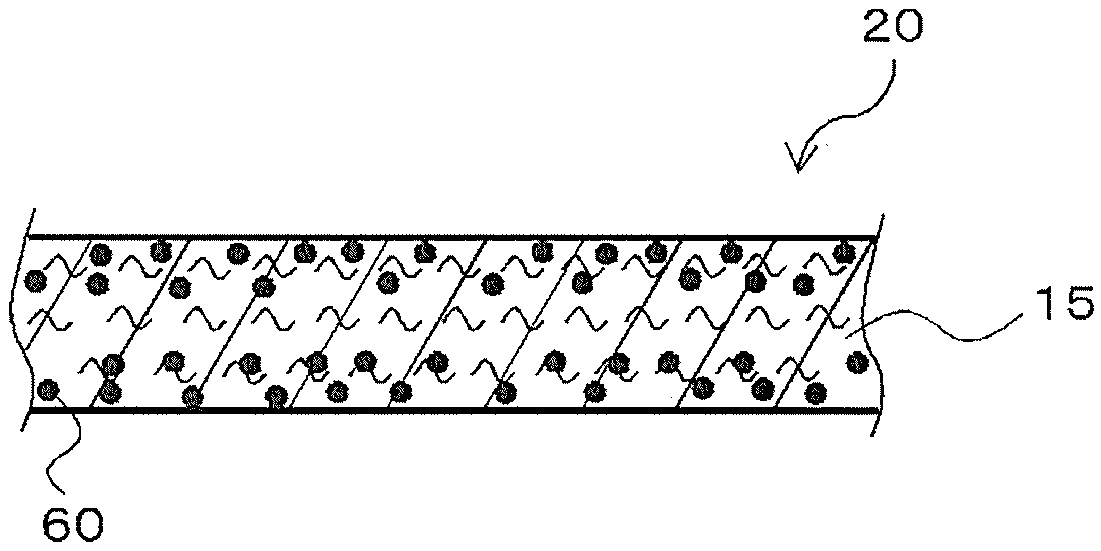
Ion scavenger for lithium ion secondary cell, liquid electrolyte, separator, and lithium ion secondary cell
An ion scavenger, secondary battery technology, applied in secondary batteries, lithium storage batteries, battery electrodes, etc., can solve the problems of battery characteristics (capacity reduction, etc., to achieve the effects of excellent safety, short circuit suppression, and excellent cycle characteristics)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0158] Hereinafter, the present invention will be specifically described based on examples. However, the present invention is not limited to the following examples.
[0159] 1. Evaluation method of ion trapping agent
[0160] (1) Moisture content rate
[0161] After the ion scavenger was vacuum dried at 150°C for 20 hours, the water content was measured by Karl Fischer method.
[0162] (2) pH measurement
[0163] Using the glass electrode type hydrogen ion concentration indicator "D-51" (model name) manufactured by Horiba Manufacturing Co., Ltd., the pH of the liquid after the ion trapping agent was added in the following (3) was measured. The measurement is performed at a measurement temperature of 25°C based on JIS Z 8802 "pH Measurement Method".
[0164] (3) Metal ion trapping ability of ion trapping agent in aqueous solution containing metal ions
[0165] ICP emission spectrometry was used to evaluate the metal ion capturing ability. The specific evaluation method is as follows.
[...
Synthetic example 1
[0173] After 0.272 mol of zirconium oxychloride octahydrate was dissolved in 850 mL of deionized water, 0.788 mol of oxalic acid dihydrate was added and dissolved. Next, while stirring the aqueous solution, 0.57 mol of phosphoric acid was added. And while stirring this mixed liquid, it refluxed at 103 degreeC for 8 hours. After cooling, the obtained precipitate was thoroughly washed with water and dried at 150°C to obtain a powder containing zirconium phosphate. As a result of analyzing the obtained zirconium phosphate, it was confirmed to be α-zirconium phosphate (H type) (hereinafter referred to as "α-zirconium phosphate (Z1)").
[0174] After boiling and dissolving the above-mentioned α-zirconium phosphate (Z1) with hydrofluoric acid-added nitric acid, the following composition formula was obtained by ICP emission spectrometry.
[0175] ZrH 2.03 (PO 4 ) 2.01 ·0.05H 2 O
[0176] In addition, the median particle diameter of α-zirconium phosphate (Z1) was measured using a laser di...
Embodiment 1
[0178] While stirring 1000 mL of a 0.1N-LiOH aqueous solution, 100 g of α-zirconium phosphate (Z1) obtained in Synthesis Example 1 was added thereto, and the mixed solution was stirred for 8 hours. After that, the precipitate was washed with water and vacuum-dried at 150°C for 20 hours to produce ZrLi 0.3 H 1.73 (PO 4 ) 2.01 ·0.06H 2 O lithium ion substituted α-zirconium phosphate. The moisture content is 0.4%. This lithium ion-substituted α-zirconium phosphate is a product obtained by replacing 1 meq / g of the total cation exchange capacity with lithium ions, and is referred to as "1meq-Li substituted α-zirconium phosphate (A1-1)" below.
[0179] Next, the 1meq-Li substituted α-zirconium phosphate (A1-1) was used as an ion scavenger, and the above-mentioned evaluations (3) and (4) were performed. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com