Application of neohesperidin in preparing medicine for preventing and treating Alzheimer's disease
A technology for senile dementia and neohesperidin, which is applied in the field of application of neohesperidin in the preparation of drugs for the prevention and treatment of senile dementia, which can solve the problems that neohesperidin has not yet been discovered, and achieve the improvement of learning and memory function decline , MDA activity reduction, memory dysfunction improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
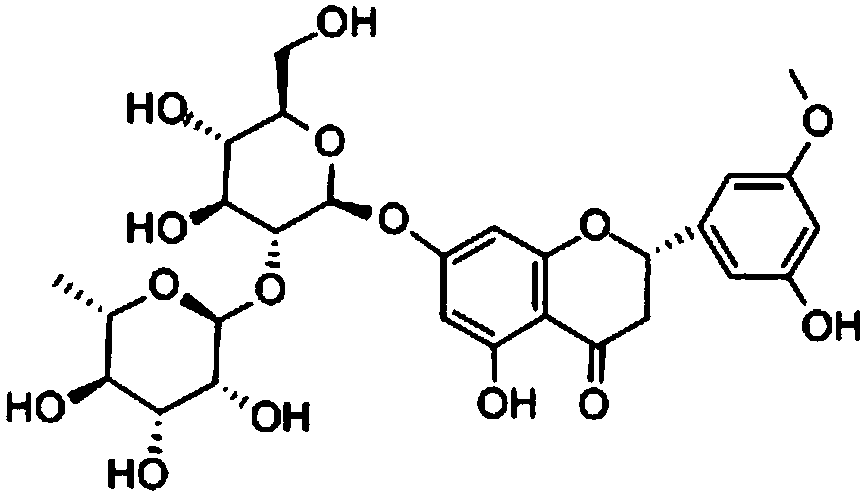
[0028] Embodiment 1: the preparation of neohesperidin
[0029] Citrus aurantium fine powder 600g, extract 3 times with 70% ethanol (crude drug: solvent weight ratio is 1: 8; 1: 6; 1: 6), combine the extracts, recover ethanol until there is no alcohol smell, add distilled water to 600ml, water The solution was sequentially extracted with petroleum ether, ethyl acetate and n-butanol, 300ml each time. The n-butanol extraction part was concentrated and dried to obtain 82g, which was put on a D101 macroporous resin column, and eluted with water, 25% ethanol, 50% ethanol and 95% ethanol in sequence, and the 50% ethanol eluted part was concentrated and dried to obtain 20% The above mixed total glycosides (47g) of neohesperidin were separated by silica gel column chromatography to obtain pure neohesperidin (95%, 20g). The chemical structure of the above pure product was determined by comparing with the IR, UV, NMR, m.p. and TLC of the standard neohesperidin.
Embodiment 2
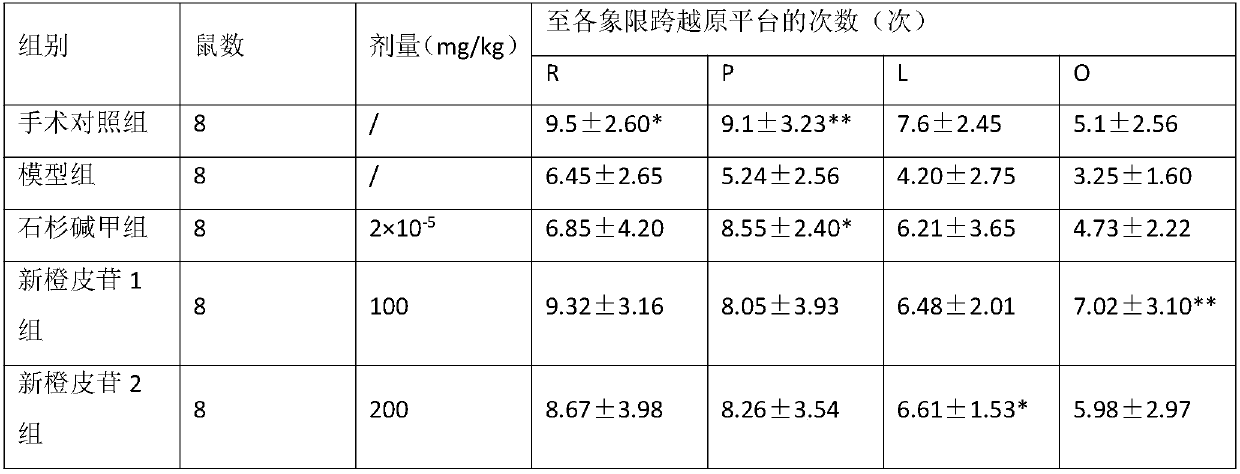
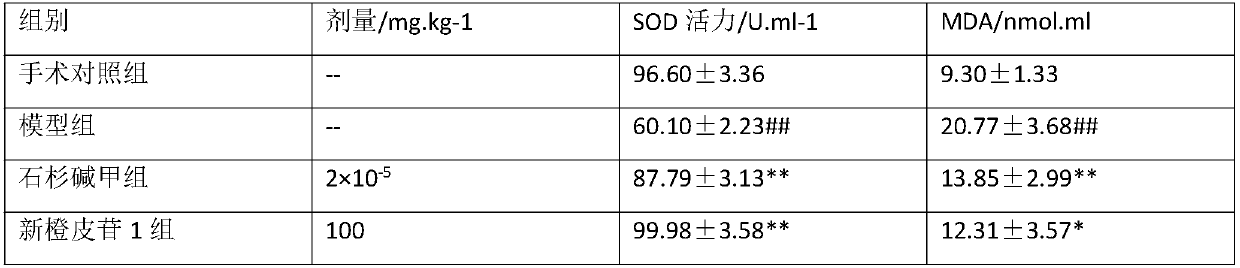
[0030] Example 2: Experimental research on the prevention and treatment effect of neohesperidin on experimental AD
[0031] 1. Experimental materials
[0032] 1.1 Experimental animals: SD rats, weighing 160-200 g, male or female, clean grade, provided by the Experimental Animal Center of Kunming Medical College.
[0033] 1.2 Drugs and reagents: Grind the pure neohesperidin obtained in 1 before the experiment, pass through a 100-mesh sieve, dissolve in fresh distilled water, make an aqueous solution with a concentration of 5%, and store it in a refrigerator at 4°C for later use; D-galactose , subpackaged by AMRSCO Company; Ibotenic acid (IBO), purchased from Sigma Company; Huperzine A Tablets (Shuangyiping), 50 μg / tablet, purchased from Shanghai Fudan Fuhua Pharmaceutical Co., Ltd.
[0034] 1.3 Experimental instruments: Rat locator, SN-2 type, made in Japan; Dental drill car, 307-6 type, Shanghai Medical Analytical Instrument Factory; Agilent1100 series HPLC instrument, Agilen...
Embodiment 3
[0068] Example 3: Effect of neohesperidin NaNO2 on memory impairment in mice
[0069] 1. Experimental materials
[0070] 1.1 Experimental animals: ICR mice, weighing 18-20g, clean grade, Kunming Pharmaceutical Group Experimental Animal Center.
[0071] 1.2 Drugs and reagents: Neohesperidin, provided by Jiangxi Qingfeng Pharmaceutical Co., Ltd., as in Example 1, crushed before the experiment, passed through a 100-mesh sieve, dissolved in fresh distilled water, and stored at 4°C as a 5% aqueous solution Standby in the refrigerator; Ginadol tablets, containing Ginkgo biloba extract 40mg / tablet, produced by Dr. Weimar Shupei Pharmaceutical Factory in Germany.
[0072] 2. Methods and results
[0073] 60 Kunming mice were randomly divided into 6 groups. After 7 days of intragastric administration in each group, all groups except the normal group were subcutaneously injected with NaNO immediately after training. 2 120mg / kg, each group was given the last dose 24 hours after the tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com