Method using co-precipitation to prepare nitrate intercalation nickel-aluminum layered double hydroxides in one step
A technology of nitrate intercalation and hydrotalcite, which is applied in the fields of chemical instruments and methods, nickel compounds, inorganic chemistry, etc., can solve the problem of not being able to prepare nitrate intercalation nickel-aluminum hydrotalcite in one step, and achieve the effect of simple and rapid preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
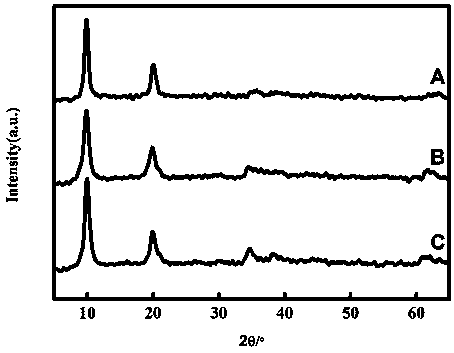
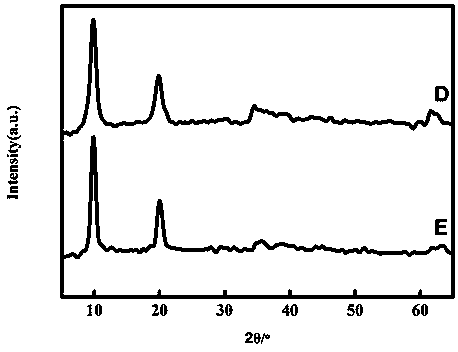
Image
Examples
Embodiment 1
[0035] (1) Use a pipette to pipette 18.00±0.01 mL of 0.2 mol L -1 sodium acetate solution into a 100 mL volumetric flask, and then add 82.00±0.01 mL 0.2 mol L -1 acetic acid solution, shake it to make it evenly mixed, and make it into 100 mL pH=4.00±0.01 acetic acid-sodium acetate buffer solution.
[0036] (2) Weigh 31.41 ± 0.01 g of nickel nitrate hexahydrate and 20.26 ± 0.01 g of aluminum nitrate nonahydrate, mix them into a beaker, add 90 ± 1 mL of deionized water into the beaker, and stir with a magnetic stirrer for 10 min. It is completely dissolved, the prepared solution (c(Ni 2+ )=1.20 mol L-1 , c(Al 3+ )=0.60 mol L -1 ) into a 250 mL hanging bottle infusion set for use.
[0037] (3) Weigh 4.00±0.01 g of sodium hydroxide into a beaker, add a small amount of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to completely dissolve it. Transfer the dissolved solution to a 100 mL volumetric flask to make 1.00mol L -1 sodium hydroxide aqueous...
Embodiment 2
[0042] (1) Pipette 49.00±0.01 mL 0.2 mol L -1 sodium acetate solution into a 100 mL volumetric flask, and then add 51.00±0.01 mL 0.2mol L -1 acetic acid solution, shake it to make it evenly mixed, and make it into 100 mL pH=4.60±0.01 acetic acid-sodium acetate buffer solution.
[0043] (2) Weigh 43.62±0.01 g of nickel nitrate hexahydrate and 18.76±0.01 g of aluminum nitrate nonahydrate, mix them into a beaker, add 100±1 mL of deionized water into the beaker, and stir for 10 min with a magnetic stirrer It is completely dissolved, the prepared solution (c(Ni 2+ )=1.50 mol L -1 , c(Al 3+ )=0.50 mol L -1 ) into a 250 mL hanging bottle infusion set for use.
[0044] (3) Weigh 4.00±0.01 g of sodium hydroxide into a beaker, add a small amount of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to completely dissolve it. Transfer the dissolved solution to a 100 mL volumetric flask to make 1.00mol L -1 sodium hydroxide aqueous solution, and then trans...
Embodiment 3
[0049] (1) Use a pipette to pipette 70.00±0.01 mL of 0.2 mol L -1 sodium acetate solution into a 100 mL volumetric flask, and then add 30.00±0.01 mL 0.2 mol L -1 acetic acid solution, shake it to make it evenly mixed, and make it into 100 mL pH=5.00±0.01 acetic acid-sodium acetate buffer solution.
[0050] (2) Weigh 34.89±0.01 g of nickel nitrate hexahydrate and 11.25±0.01 g of aluminum nitrate nonahydrate, mix them into a beaker, add 120±5 mL of deionized water into the beaker, and stir it with a magnetic stirrer for 10 min. It is completely dissolved, the prepared solution (c(Ni 2+ )=1.00 mol L -1 , c(Al 3+ )=0.25 mol L -1 ) into a 250 mL hanging bottle infusion set for use.
[0051] (3) Weigh 4.00±0.01 g of sodium hydroxide into a beaker, add a small amount of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to completely dissolve it. Transfer the dissolved solution to a 100 mL volumetric flask to make 1.00mol L -1 sodium hydroxide aqueous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com