Nisin polylysine antibacterial nano particles and preparation method thereof
A nanoparticle and polylysine technology, which is applied in the field of food antibacterial additives, can solve the problems of reducing stability and biological activity, and achieve the effects of good product stability, special product structure and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The specific implementation of the present invention is illustrated by the following examples, but the protection content of the present invention is not limited thereto. The preparation of embodiment 1Nisin polylysine antibacterial nanoparticles
[0024] 1 Experimental materials
[0025] Nisin potency 1100IU / mg Luoyang Qihong Biotechnology Co., Ltd.
[0026] γ-PGA Mw 50,000-100,000 Shandong Freda Biotechnology Co., Ltd.
[0027] PLL Mw 150,000-300,000 Shanghai Yuanye Biotechnology Co., Ltd.
[0028] 2 Experimental methods
[0029] The preparation steps of Nisin polylysine antibacterial nanoparticles are as follows:
[0030] ①Use deionized water to prepare Nisin solution (2.0mg / mL), γ-PGA solution (3.0mg / mL) and PLL solution (2.0mg / mL).
[0031] ② Mix Nisin solution (2.0mg / mL) and γ-PGA solution (3.0mg / mL) in equal volumes, and stir the mixture on a magnetic stirrer (200rpm) for 2h at room temperature.
[0032] ③Add an equal volume (equal volume to Nisin solution)...
Embodiment 2
[0034] The characterization of embodiment 2Nisin polylysine antibacterial nanoparticles
[0035] 1 Experimental materials
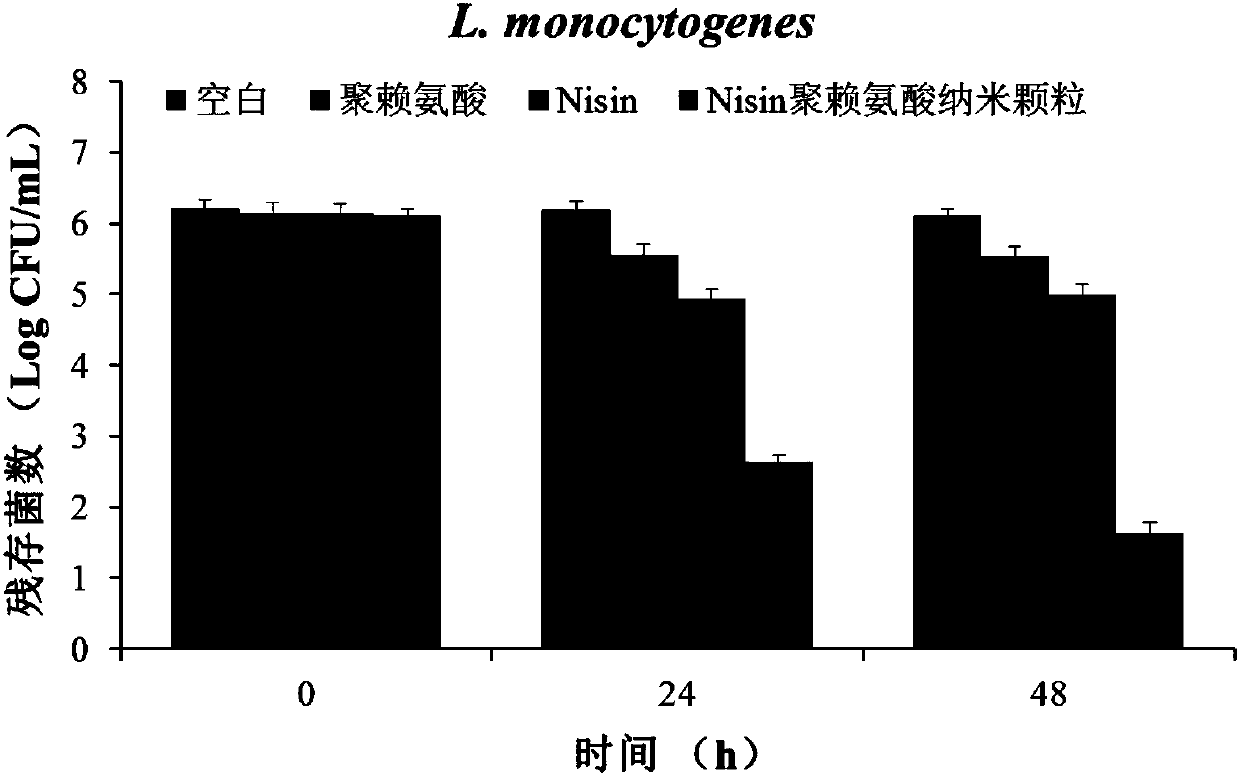
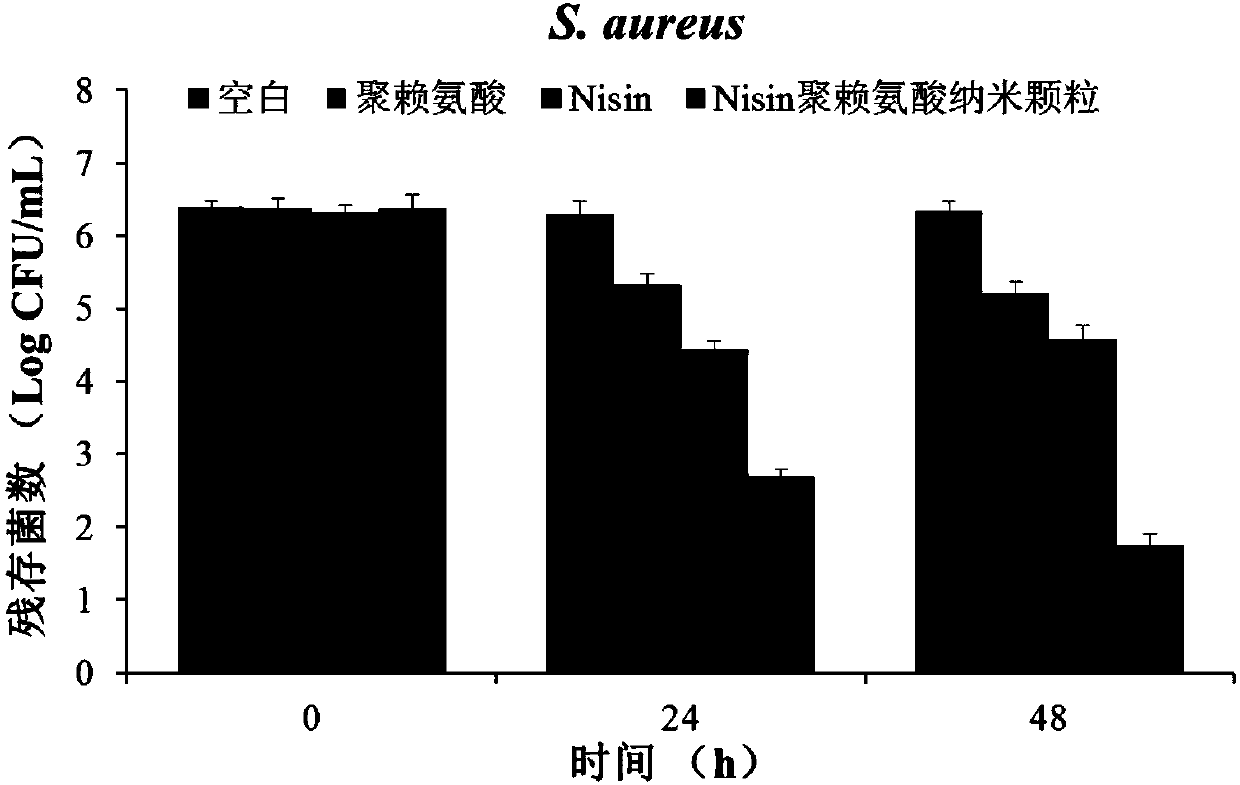
[0036] Nisin polylysine antibacterial nanoparticles
[0037] 2 Experimental materials and instruments
[0038]
[0039] 3 Experimental methods
[0040] ①The particle size and polydispersity index (PDI) of Nisin polylysine antibacterial nanoparticles were measured by laser particle size analyzer (Nano ZS90). The Zeta potential of antibacterial nanoparticles was measured with a potentiometer (Nano ZS90Zeta).
[0041] ②The encapsulation efficiency of Nisin in the antibacterial nanoparticles was determined indirectly by BCA method trace protein detection kit. During the preparation of Nisin polylysine antibacterial nanoparticles, the supernatant was collected, and the concentration of Nisin in the supernatant (that is, the concentration of unencapsulated Nisin in the nanoparticles) was determined using a BCA method trace protein detection kit. Utilize...
Embodiment 3
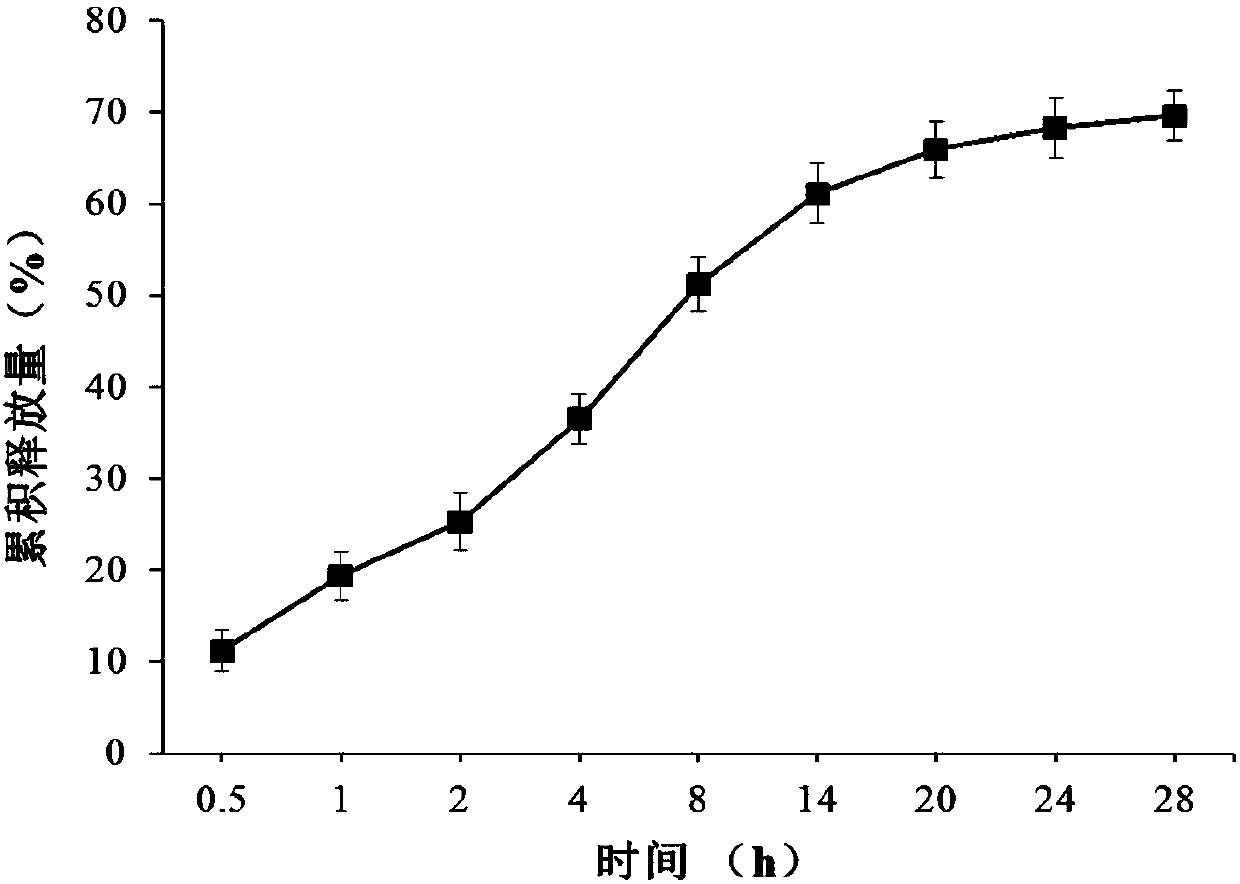
[0049] Nisin release performance evaluation in embodiment 3Nisin polylysine antibacterial nanoparticles
[0050] 1 Experimental materials
[0051] Nisin polylysine antibacterial nanoparticles
[0052] 2 experimental equipment
[0053] BCA Trace Protein Detection Kit / Nanjing Jiancheng Bioengineering Co., Ltd.
[0054] Dialysis bag 7000Da Beijing Solaibao Technology Co., Ltd.
[0055] 3 Experimental methods
[0056] Weigh a certain amount of Nisin polylysine antibacterial nanoparticles, put it into a dialysis bag (molecular cut-off 7000Da), then put the dialysis bag in 100.0mL PBS, and place it on a magnetic stirrer to rotate slowly (100 rpm) . At regular intervals, 500 μL of samples were taken out, and an equal amount of PBS was added after sampling to maintain a constant solution volume. The sample taken out was centrifuged (6000rpm / min) for 15min, and the supernatant was taken, and the concentration of Nisin in the supernatant was determined using a BCA method trace p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com