Lithium nickel cobalt aluminum oxide positive electrode material and preparation method therefor
A technology of nickel-cobalt lithium aluminate and positive electrode materials, which is applied in the field of lithium-ion batteries, can solve problems such as phase separation, anti-dissolution, and complex processes, and achieve the effects of high tap density, uniform composition, and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The invention provides a method for preparing a nickel-cobalt lithium aluminate cathode material, comprising the following steps:
[0032] (1) will include Ni 2+ 、Co 2+ and Al 3+ The metal salt solution, the complexing agent solution and the precipitating agent solution are mixed, and the nickel-cobalt-aluminum precursor is prepared by the liquid phase controlled crystallization method; the complexing agent in the complexing agent solution includes fluoride, alcohol ammonia compound, phosphoric acid compound and one or more of carbonyl compounds;
[0033] (2) The nickel-cobalt-aluminum precursor prepared in step (1) is mixed with a solid-phase lithium source and then sintered to obtain a nickel-cobalt lithium aluminate positive electrode material.
[0034] The present invention will include Ni 2+ 、Co 2+ and Al 3+ The metal salt solution, the complexing agent solution and the precipitating agent solution are mixed, and the nickel-cobalt-aluminum precursor is prepar...
Embodiment 1
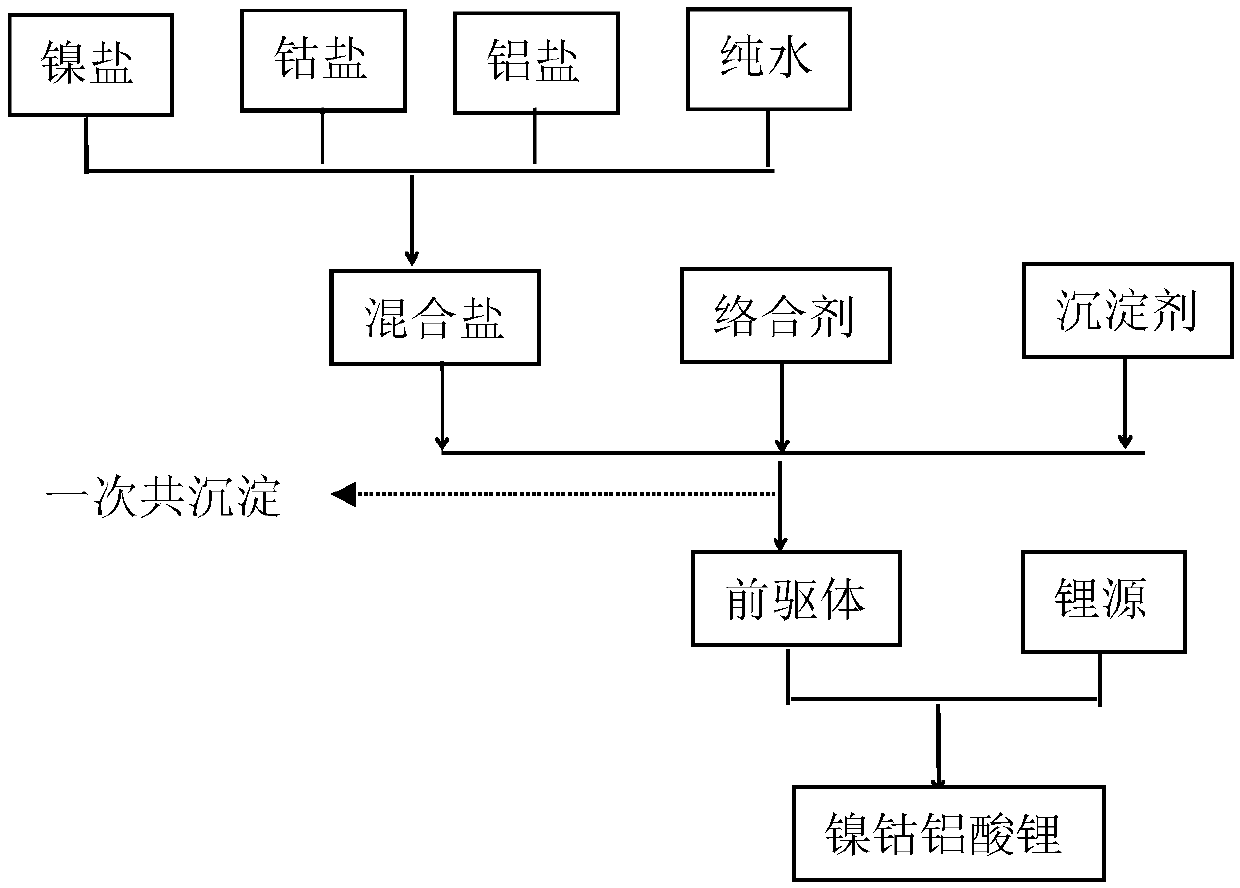
[0061] according to figure 1 The flow chart shown prepares nickel cobalt lithium aluminate cathode material:
[0062] (1) Preparation of the precursor: the total concentration of the prepared metal ions is 10mol / L and Ni 2+ 、Co 2+ with Al 3+The metal sulfate solution with a molar ratio of 5:4:1, the NaOH solution with a molar concentration of 5mol / L, and the ammonium tartrate solution with a molar concentration of 10mol / L, according to the metal salt ions in the metal salt solution and the complexing agent solution The molar ratio of complexing agent 0.01:2, weigh metal sulfate solution and ammonium tartrate solution, by controlling the consumption of sodium hydroxide solution, make the pH value of reaction system be 8, metal sulfate solution, NaOH solution and ammonium tartrate The solution is simultaneously pumped into the reactor in a co-current manner, the pH of the reaction system is controlled to be 8.0, and the temperature of the solution in the reactor is 40° C. to ...
Embodiment 2
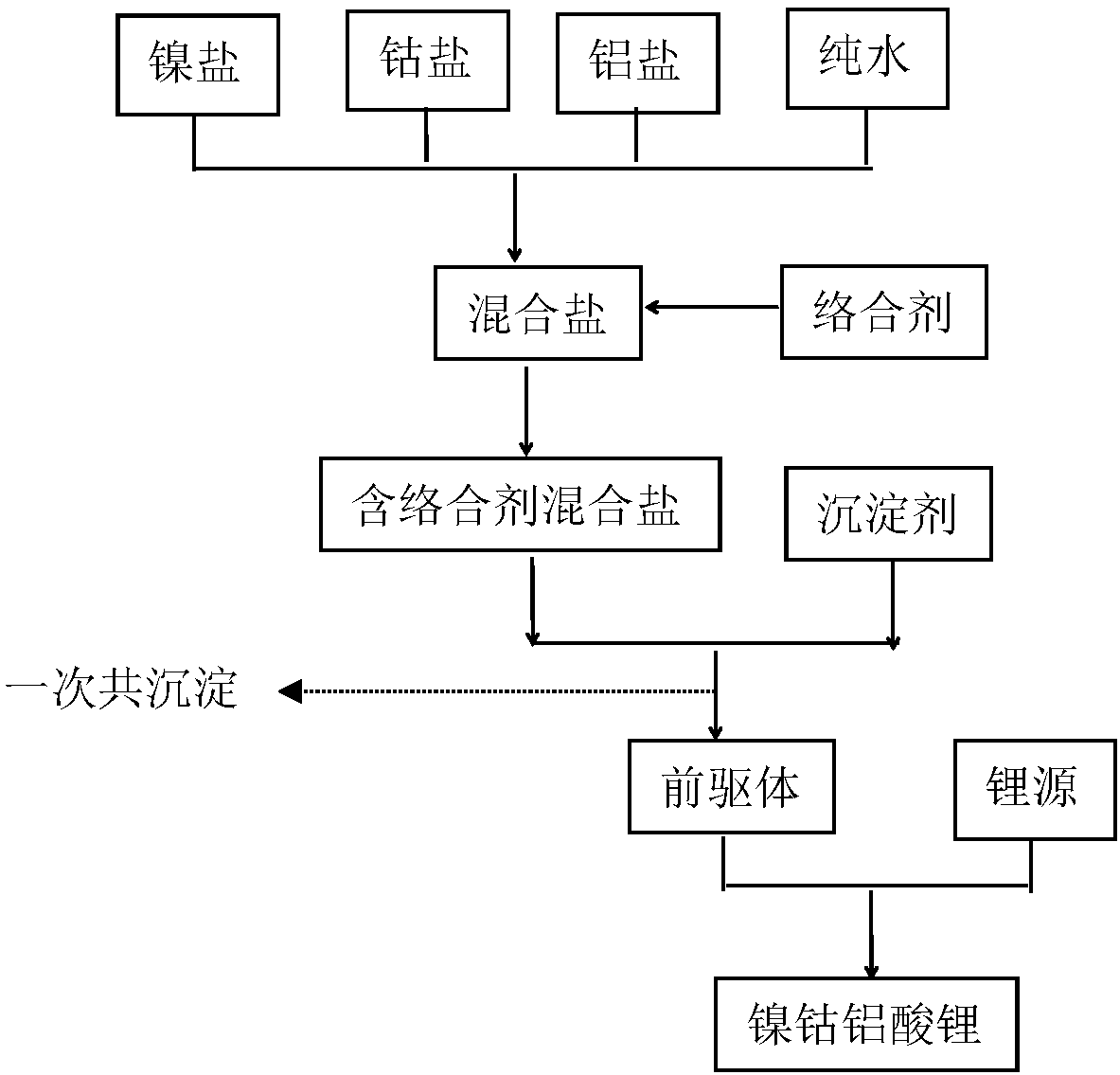
[0070] according to figure 1 The flow chart shown prepares nickel-cobalt lithium aluminate cathode material:
[0071] (1) Preparation of the precursor: prepare the total concentration of metal ions to be 3mol / L and Ni 2+ 、Co 2+ with Al 3+ The metal sulfate solution with a molar ratio of 75:20:5, the NaOH solution with a molar concentration of 4mol / L, the mixed complexing agent solution of sodium hexametaphosphate and sodium nitrilotriacetate with a molar concentration of 1mol / L each, according to the metal The mol ratio of metal salt ion in the sulfate solution and complexing agent solution 5:2, weigh metal sulfate solution and complexing agent solution, by controlling the consumption of sodium hydroxide solution, make the pH value of reaction system was 11.5, the metal sulfate solution and complexing agent solution were simultaneously pumped into the reactor in a co-current manner, the pH of the reaction system was controlled to be 11.5, and the temperature of the solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com