Targeted Nanocarriers For Targeted Drud Delivery Of Gene Therapeutics
A nano-carrier and drug technology, applied in the medical field, can solve problems that hinder the effectiveness of gene targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
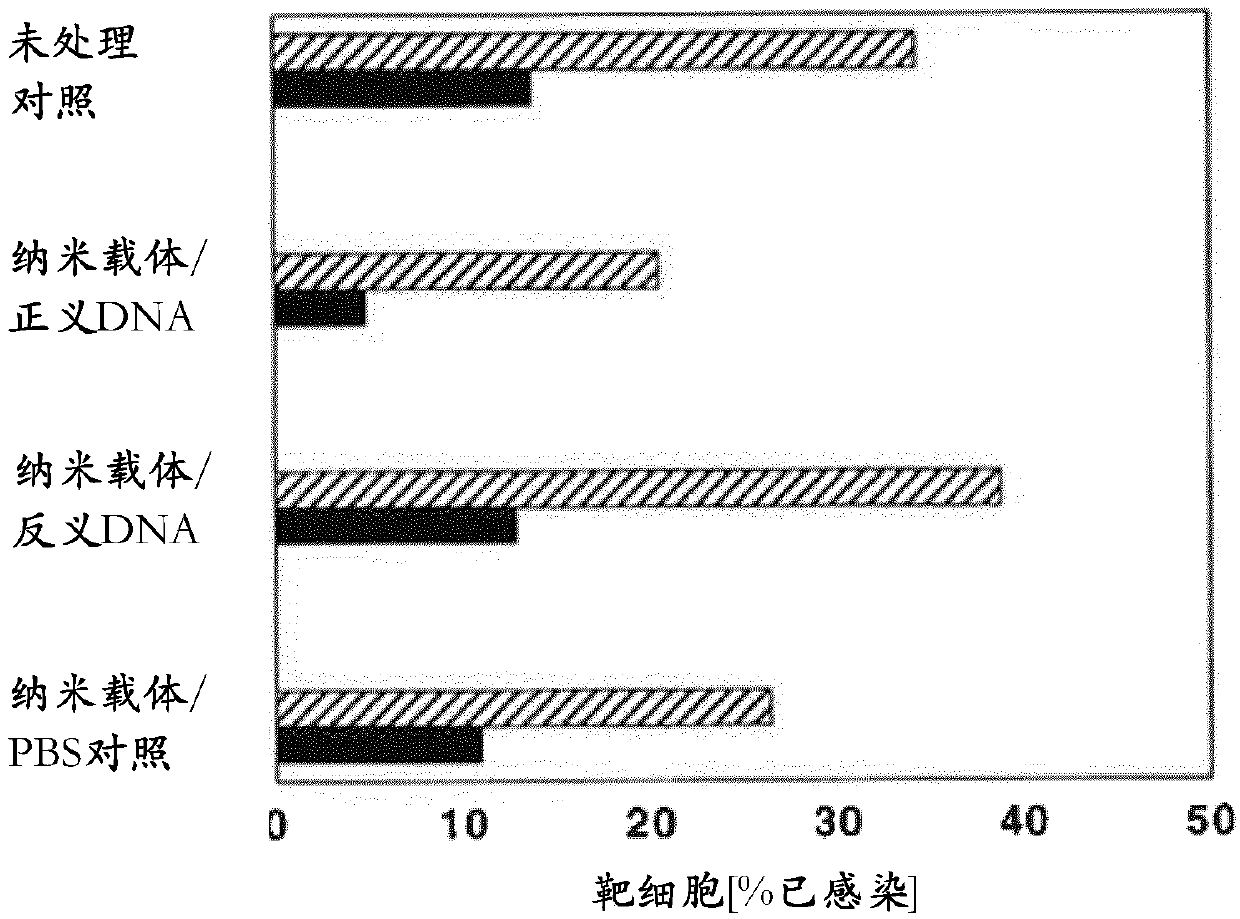
[0076]In the case of HIV-1, the gene-therapy approach described herein is to inhibit the proliferation of the virus by (i) genome editing via plasmid-encoded TALEN activity, or (ii) via sense or antisense oligonucleotides. Nucleotides block selected viral genes. Infection by HIV-1 can be used as an example, since the viral genome will integrate into the host genome as a so-called HIV-1 provirus. The same method, or variants thereof, can be transferred or adapted to other indications.
example 1
[0078] Example 1: Targeted Lipid-Based Nanocarriers
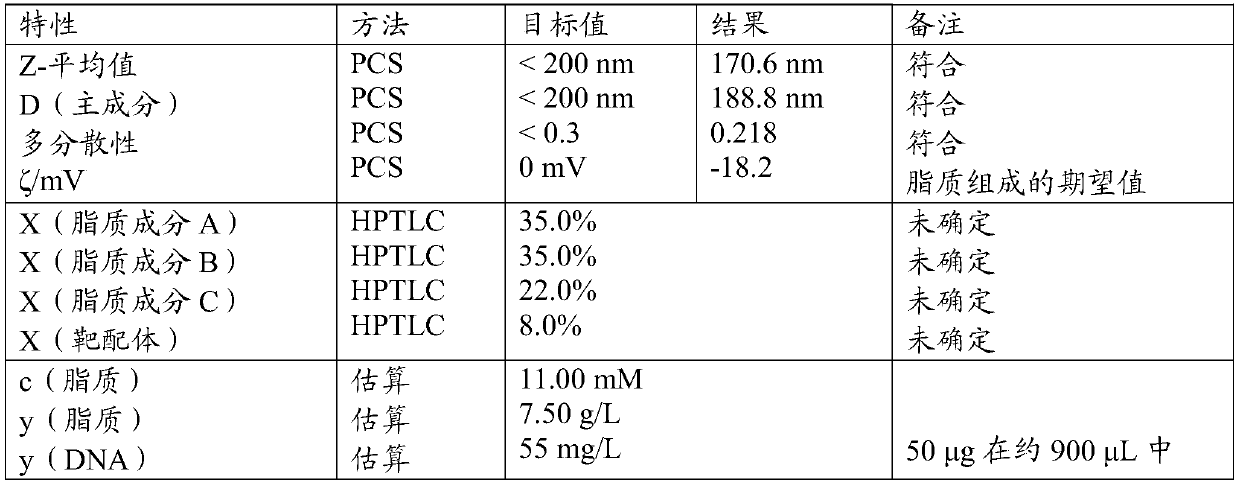
[0079] According to the basic protocol previously published (Gieseler Rk et al., March 21, 2005, WO / 2005 / 092288; Gieseler Rk et al., Scand J Immunol [Scandinavian Immunology Journal] 2004;59:415- 24. PMID15140050) Formulation of nanocarriers. However, a modification was made to the protocol in that the density of the targeting anchors was increased to 8% of the surface density of the fucose-derived anchors used to localize cells via C-type lectin receptors expressed on the cell surface, or 8% of the surface density of galactose-derived anchors for targeting cells via asialoglycoprotein receptors expressed on the cell surface. In addition, liposome compositions can be modified using: phosphatidylcholine (PC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-di Oleoyl-sn-glyceryl-3-phosphoethanolamine (DOPE), 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), cholesteryl hemisuccinate (CHEMS), soybean phosphatidyl Choli...
example 2
[0080] Example 2: Encapsulation of plasmid DNA in nanocarriers
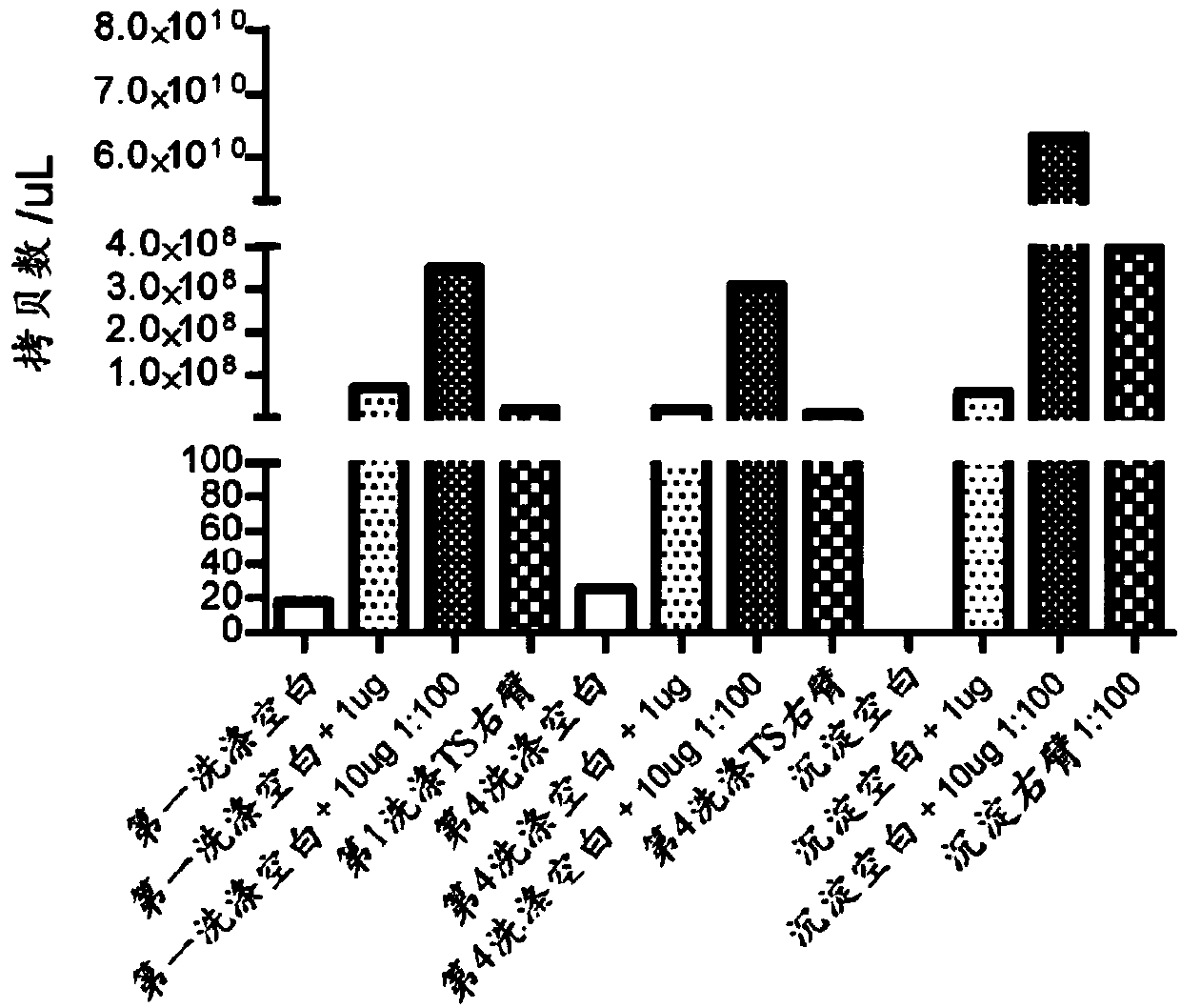
[0081] The DNA in the form of a plasmid (p-DNA) encapsulates the gene encoding green fluorescent protein (GFP) as a reporter gene or the sequence encoding TALEN. As a basis for previous protocols, encapsulation of DNA was performed initially (Pupo E et al J ContrRelease 2005;104:379-96; Bailey AL and Sullivan SM. Biochim BiophysActa 2000;1468:239-52). However, a modification was made to the protocol in that the formulation parameters of multilamellar vesicles (MLV) to produce small unilamellar vesicles (SUVs) were compared with the high pressure homogenization parameters (extrusion: 50 nm or 100 nm) Revise. Also, as a way to increase the packing ratio, speed mixing is introduced to ultimately leave an acceptable payload packing ratio. Unencapsulated DNA payloads were removed by dialysis from phosphate buffer in which nanocarriers were dissolved. However, omitting the dialysis step may be a future option, sinc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com