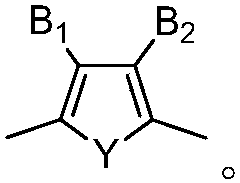
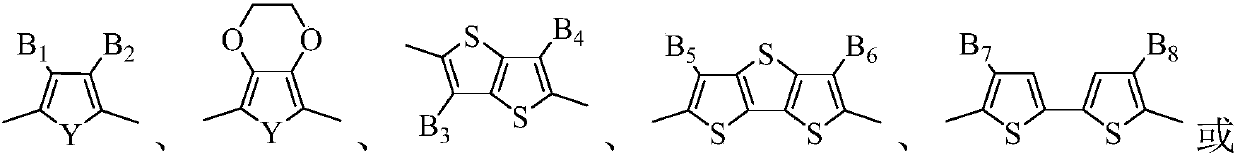
Organic photosensitizer and preparation method and application thereof
A photosensitizing dye and organic technology, applied in the field of organic photosensitizing dyes and their preparation, can solve the problems of small short-circuit current density, small molar absorption coefficient, low molar absorption coefficient and short-circuit current density, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: the synthesis of organic photosensitive dye (POZ-PTZ-TC)
[0079] In the synthesis of organic photosensitive dyes (POZ-PTZ-TC), intermediate p and intermediate i need to be prepared first, and the preparation method of intermediate p, intermediate i, and intermediate p and intermediate i are as follows The sequence for preparing organic photosensitive dyes (POZ-PTZ-TC) is described:
[0080] 1. Preparation of intermediate p
[0081] (1) Synthesis of intermediate m
[0082]
[0083] Add phenoxazine and 1,6-dibromohexane (molar ratio = 1:8), sodium hydride, and ethylene glycol dimethyl ether into a three-necked flask, fill with nitrogen, stir at room temperature for 24 h, wash the reaction mixture with water, and It was extracted with methyl chloride, dried with anhydrous, concentrated by rotary evaporation, and a colorless liquid was obtained through silica gel column chromatography with petroleum ether as eluent, with a yield of 67.1%.
[0084] NMR c...
Embodiment 2
[0144] Embodiment 2: the ultraviolet-visible absorption spectrum test of dyestuff (POZ-PTZTC) and contrast dyestuff (PTZSC-RD)
[0145] The synthetic organic photosensitive dyes R-PTZSC and POZ-PTZ-TC were formulated into 2×10 -5 The dichloromethane-tetrahydrofuran solution of mol / L adopts METASH UV 5200PC ultraviolet spectrophotometer to carry out the test of absorption spectrum, and test result is as follows figure 1 As shown in the figure, the abscissa represents the absorption wavelength in nanometers, and the ordinate represents the molar absorptivity coefficient in units of 10 4 m -1 cm -1 .
[0146] pass figure 1 It can be seen that the molar absorptivity at the maximum absorption wavelength of the organic photosensitive dye POZ-PTZ-TC with phenothiazine and phenoxazine as electron donors and containing three independent light-harvesting units and multiple adsorption groups is 8.86×10 4 m -1 cm -1 , the molar absorptivity at the maximum absorption wavelength of t...
Embodiment 3
[0147] Example 3: Dye-sensitized solar cell assembled with dye (POZ-PTZ-TC) and contrast dye (R-PTZSC) and battery performance test
[0148] 1. Assembly of dye-sensitized solar cells (dye: POZ-PTZ-TC)
[0149] (1) Pretreatment of conductive glass
[0150] Cut the conductive glass into a size of 5.5×7.5cm, then ultrasonically clean the cut conductive glass with detergent, acetone, deionized water, and ethanol for 30 minutes, and finally dry it for later use.
[0151] (2) Preparation of battery photoanode
[0152] Place the conductive glass with the conductive side facing up, and screen-print the TiO 2 The paste is printed on conductive glass, and TiO with a thickness of 2 μm can be prepared in one printing 2 film, which will then be attached with TiO 2 The conductive glass of the film was dried in an oven at 125°C, and repeated 5 times to obtain TiO with a total thickness of 10 μm. 2 membrane. Using the same method and then on the 10μm TiO 2 Large particles of TiO with a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Short circuit current density | aaaaa | aaaaa |
| Open circuit voltage | aaaaa | aaaaa |
| Short circuit current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com