Arylamine compound and organic electroluminescent element
A technology of arylamine compound and organic layer, applied in the field of organic electroluminescent devices, can solve the problems of insufficient improved characteristics, high luminous efficiency, low driving voltage, etc., and achieve excellent electron blocking ability, high luminous efficiency, driving The effect of voltage reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
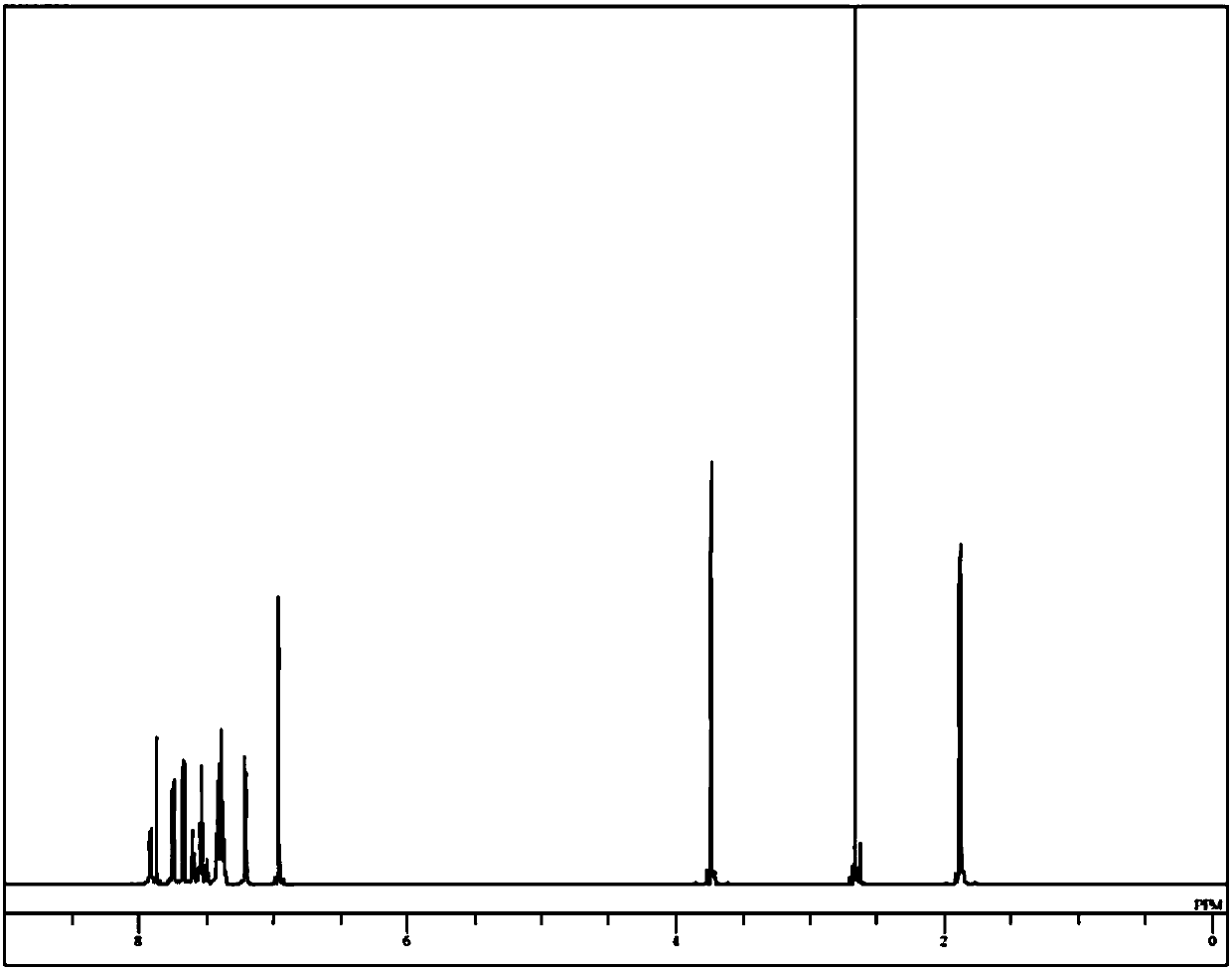
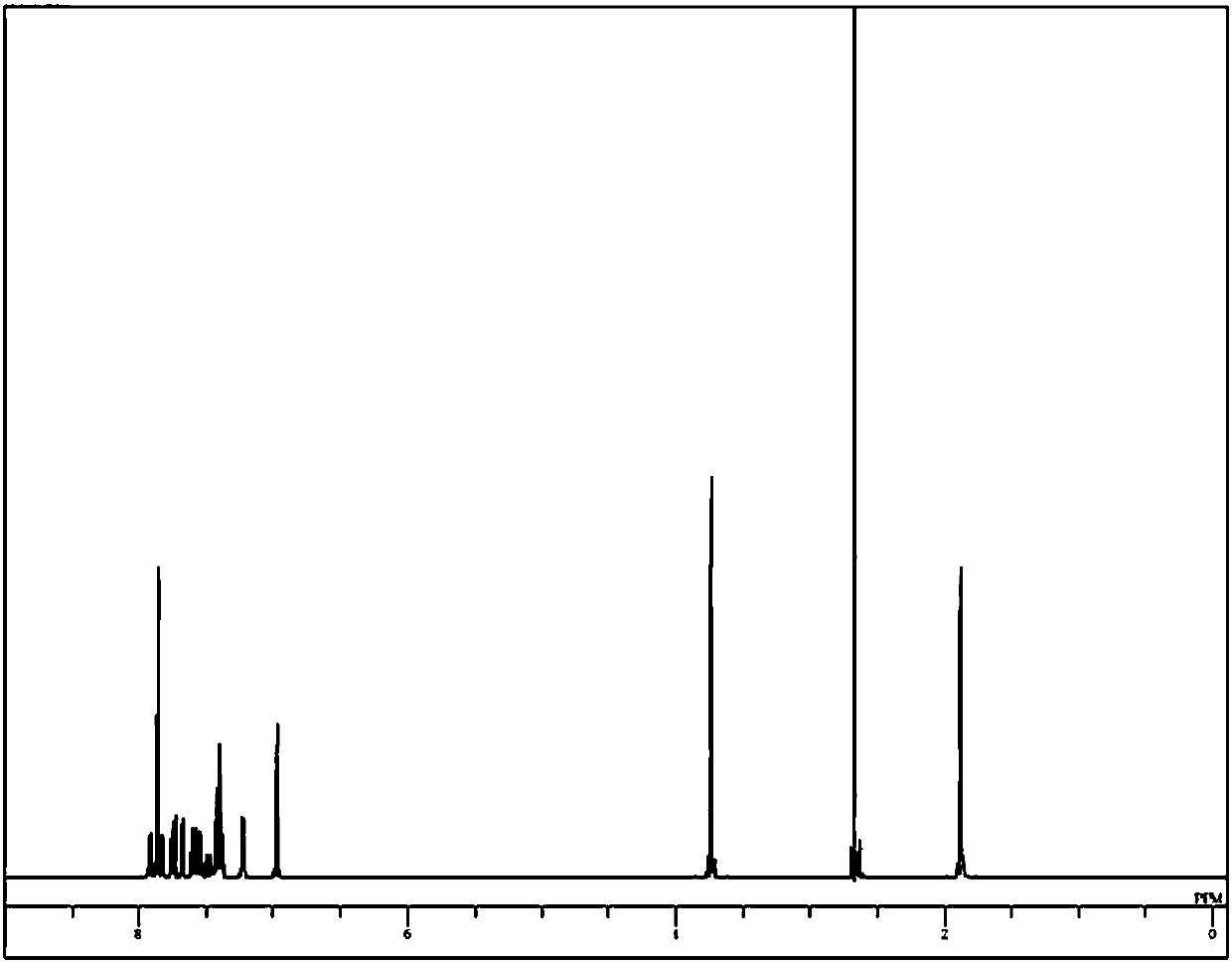
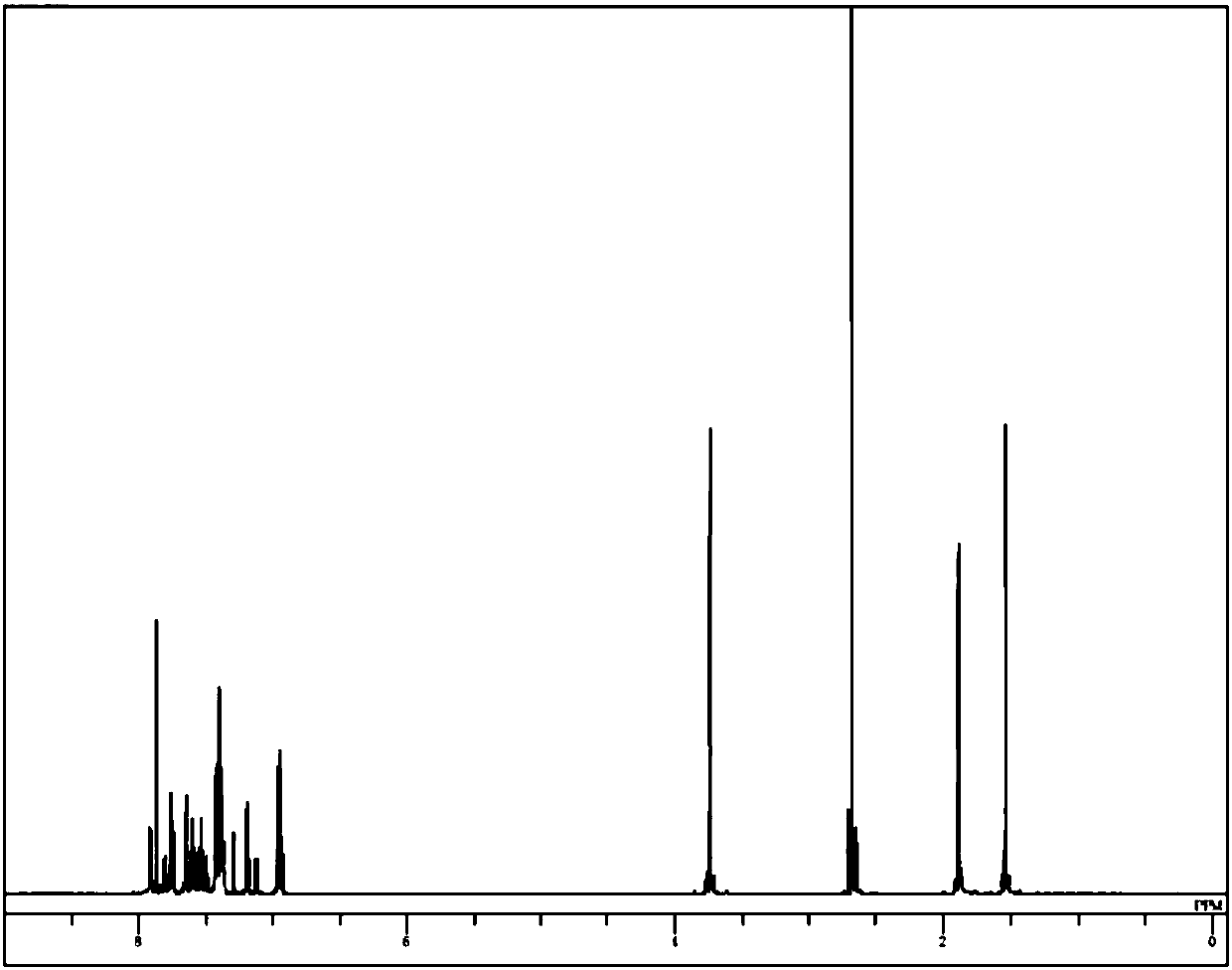
Image
Examples
Embodiment approach
[0104] Ar 1 and Ar 2 may be the same or different, and each represents, preferably, an aromatic hydrocarbon group or a condensed polycyclic aromatic group not having a heteroatom (for example, nitrogen atom, oxygen atom or sulfur atom); more preferably, phenyl, naphthalene phenanthrenyl or fluorenyl; particularly preferably substituted phenyl or substituted fluorenyl. As the substituent for phenyl, phenyl, biphenyl, terphenyl, naphthyl, phenanthrenyl or fluorenyl are preferred. As the substituent for the fluorenyl group, methyl group or phenyl group is preferable.
[0105] From a synthetic point of view, if Ar 1 or Ar 2 With a substituent, the substituted Ar 1 or Ar 2 Preferably having L, Ar combined with the benzene ring and bound to the benzene ring 3 、Ar 4 and R 1 ~R 3 The composition of the structure is different.
[0106] Ar 3 and Ar 4 may be the same or different, and each represents, preferably, an aromatic hydrocarbon group or a fused pol...
Embodiment 1
[0206]
[0207] Synthesis of N,N-bis(biphenyl-4-yl)-N-[4-{(2,4,6-triphenyl)phenyl}phenyl]amine:
[0208] (process 1)
[0209] Into a nitrogen-purged reaction vessel
[0210] 1,3,5-Triphenylbenzene 50.7g, and
[0211] Chloroform 500ml.
[0212] then join
[0213] Bromine 29.1g,
[0214] And the mixture was stirred at room temperature for 16 hours to prepare a reaction liquid. To the reaction liquid, a saturated aqueous solution of sodium sulfite was added, and the mixture was stirred. Then, a liquid separation operation was performed and an organic layer was collected. The organic layer was dehydrated over magnesium sulfate, and then concentrated under reduced pressure to obtain a crude product. Hexane was added to the crude product, and the mixture was dispersed and washed. As a result, 55.0 g (yield 86%) of 2-bromo-1,3,5-triphenylbenzene was obtained as a white powder.
[0215] (process 2)
[0216] Into a nitrogen-purged reaction vessel
[...
Embodiment 2
[0235]
[0236] N-(biphenyl-4-yl)-N-(1,1':4',1"-terphenyl-4-yl)-N-[4-{(2,4,6-triphenyl) Synthesis of phenyl}phenyl]amine:
[0237] Into a nitrogen-purged reaction vessel
[0238]
[0239] Nitrogen was passed through the vessel for 30 minutes to obtain a mixed liquor. Add to the mixture
[0240] Tetrakis(triphenylphosphine)palladium 2.7g,
[0241] Heating was followed, and the mixture was stirred at 73° C. for 5 hours, thereby obtaining a reaction liquid. To the reaction liquid, 100 ml of water was added, and the precipitated solid was collected by filtration. To the resulting solid, o-dichlorobenzene was added, and the mixture was heated until the solid dissolved. Further, silica gel was added, and the mixture was stirred, followed by hot filtration. The filtrate was concentrated under reduced pressure, and the precipitated solid was collected by filtration. As a result, 20.1 g (yield 43%) of N-(biphenyl-4-yl)-N-(1,1':4',1"-terphenyl-4-yl)amine was o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com