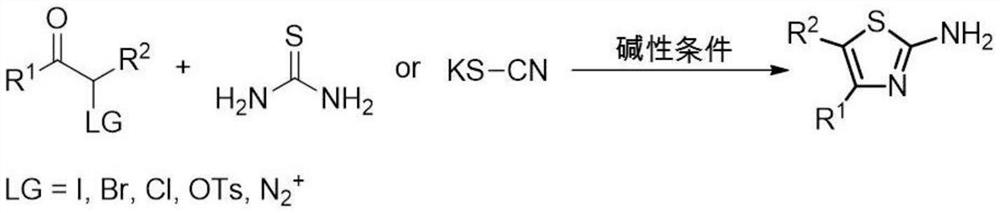
A method for synthesizing 2-aminothiazole derivatives
An aminothiazole and derivative technology, which is applied in the field of synthesizing 2-aminothiazole derivatives, can solve the problems of high catalyst cost, heating under reaction conditions, complicated preparation of raw materials, etc., and achieves the effects of easy availability of reaction reagents, widening scope and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The method for synthesizing 2-amino-4-phenyl-5-cyanothiazole (III-a), comprises the steps:
[0035] Add 3-carbonyl-3-phenylpropionitrile (I-a) (145 mg) and thiourea (II) (152 mg) to methanol (5 mL) at room temperature, then add tert-butanol peroxide (288 μL) and azobis Isobutyronitrile (33 mg), stirred at room temperature for 2 hours. Add saturated aqueous sodium bicarbonate solution (50mL) after the reaction, extract with ethyl acetate, the organic phase is dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain the crude product, and the crude product is subjected to column chromatography (ethyl acetate:petroleum ether (v / v)=3:7) separation and purification to obtain 175 mg of white solid 2-amino-4-phenyl-5-cyanothiazole (III-a), yield: 87%.
[0036] 1 H NMR (600MHz, DMSO-d 6 )δ8.25(s,2H),7.93(d,J=7.2Hz,2H),7.56–7.39(m,3H). 13 C NMR (150MHz, DMSO-d 6 )δ170.6, 161.0, 132.5, 130.0, 128.8, 127.4, 115.3, 83.6.
Embodiment 2
[0038] The method for synthesizing 2-amino-4-(4-methoxyphenyl)-5-cyanothiazole (III-b), comprises the steps:
[0039] 3-Carbonyl-3-(4-methoxyphenyl)propionitrile (I-b) (175 mg) and thiourea (II) (152 mg) were added to methanol (5 mL) at room temperature, and peroxygen was added to the reaction tert-butanol (288 μL) and azobisisobutyronitrile (33 mg), stirred at room temperature for 2 hours. Add saturated aqueous sodium bicarbonate solution (50mL) after the reaction, extract with ethyl acetate, the organic phase is dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain the crude product, and the crude product is subjected to column chromatography (ethyl acetate:petroleum ether (v / v)=3:7) separation and purification to obtain 213 mg of white solid 2-amino-4-(4-methoxyphenyl)-5-cyanothiazole (III-b), yield: 92%.
[0040] 1 H NMR (600MHz, DMSO-d 6 )δ8.19(s,2H),7.90(d,J=9.0Hz,2H),7.07(d,J=8.4Hz,2H),3.82(s,3H). 13 C NMR (150MHz, DMSO-d 6 )δ170.3,...
Embodiment 3
[0042] A method for synthesizing 2-amino-4-(4-chlorophenyl)-5-cyanothiazole (III-c), comprising the steps of:
[0043] 3-Carbonyl-3-(4-chlorophenyl)propionitrile (I-c) (180 mg) and thiourea (II) (152 mg) were added to methanol (5 mL) at room temperature, and tert-butyl peroxide was added to the reaction Alcohol (288 μL) and azobisisobutyronitrile (33 mg) were stirred at room temperature for 2 hours. Add saturated aqueous sodium bicarbonate solution (50mL) after the reaction, extract with ethyl acetate, the organic phase is dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain the crude product, and the crude product is subjected to column chromatography (ethyl acetate:petroleum ether (v / v)=3:7) separation and purification to obtain 191 mg of white solid 2-amino-4-(4-chlorophenyl)-5-cyanothiazole (III-c), yield: 81%.
[0044] 1 H NMR (600MHz, DMSO-d 6 )δ8.27(s,2H),7.93(d,J=9.0Hz,2H),7.60(d,J=8.4Hz,2H). 13 C NMR (150MHz, DMSO-d 6 )δ170.7, 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com